Development and Function of Secondary and Tertiary Lymphoid Organs in the Small Intestine and the Colon
- PMID: 27656182
- PMCID: PMC5011757
- DOI: 10.3389/fimmu.2016.00342
Development and Function of Secondary and Tertiary Lymphoid Organs in the Small Intestine and the Colon
Abstract
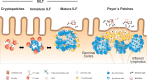
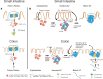
The immune system of the gut has evolved a number of specific lymphoid structures that contribute to homeostasis in the face of microbial colonization and food-derived antigenic challenge. These lymphoid organs encompass Peyer's patches (PP) in the small intestine and their colonic counterparts that develop in a programed fashion before birth. In addition, the gut harbors a network of lymphoid tissues that is commonly designated as solitary intestinal lymphoid tissues (SILT). In contrast to PP, SILT develop strictly after birth and consist of a dynamic continuum of structures ranging from small cryptopatches (CP) to large, mature isolated lymphoid follicles (ILF). Although the development of PP and SILT follow similar principles, such as an early clustering of lymphoid tissue inducer (LTi) cells and the requirement for lymphotoxin beta (LTβ) receptor-mediated signaling, the formation of CP and their further maturation into ILF is associated with additional intrinsic and environmental signals. Moreover, recent data also indicate that specific differences exist in the regulation of ILF formation between the small intestine and the colon. Importantly, intestinal inflammation in both mice and humans is associated with a strong expansion of the lymphoid network in the gut. Recent experiments in mice suggest that these structures, although they resemble large, mature ILF in appearance, may represent de novo-induced tertiary lymphoid organs (TLO). While, so far, it is not clear whether intestinal TLO contribute to the exacerbation of inflammatory pathology, it has been shown that ILF provide the critical microenvironment necessary for the induction of an effective host response upon infection with enteric bacterial pathogens. Regarding the importance of ILF for intestinal immunity, interfering with the development and maturation of these lymphoid tissues may offer novel means for manipulating the immune response during intestinal infection or inflammation.
Keywords: cryptopatch; isolated lymphoid follicles; large intestine; lymphoid tissue inducer cells; small intestine; tertiary lymphoid organs.
Figures
References
-
- Chikly B. Who discovered the lymphatic system. Lymphology (1997) 30:186–93. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

