Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis
- PMID: 27656400
- PMCID: PMC5021673
- DOI: 10.1016/j.molmet.2016.06.013
Lipolysis sensation by white fat afferent nerves triggers brown fat thermogenesis
Abstract
Objective: Metabolic challenges, such as a cold environment, stimulate sympathetic neural efferent activity to white adipose tissue (WAT) to drive lipolysis, thereby increasing the availability of free fatty acids as one source of fuel for brown adipose tissue (BAT) thermogenesis. WAT is also innervated by sensory nerve fibers that network to metabolic brain areas; moreover, activation of these afferents is reported to increase sympathetic nervous system outflow. However, the endogenous stimuli sufficient to drive WAT afferents during metabolic challenges as well as their functional relation to BAT thermogenesis remain unknown.
Method: We tested if local WAT lipolysis directly activates WAT afferent nerves, and then assessed whether this WAT sensory signal affected BAT thermogenesis in Siberian hamsters (Phodopus sungorus).
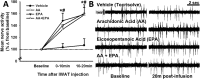
Results: 2-deoxyglucose, a sympathetic nervous system stimulant, caused β-adrenergic receptor dependent increases in inguinal WAT (IWAT) afferent neurophysiological activity. In addition, direct IWAT injections of the β3-AR agonist CL316,243 dose-dependently increased: 1) phosphorylation of IWAT hormone sensitive lipase, an indicator of SNS-stimulated lipolysis, 2) expression of the neuronal activation marker c-Fos in dorsal root ganglion neurons receiving sensory input from IWAT, and 3) IWAT afferent neurophysiological activity, an increase blocked by antilipolytic agent 3,5-dimethylpyrazole. Finally, we demonstrated that IWAT afferent activation by lipolysis triggers interscapular BAT thermogenesis through a neural link between these two tissues.
Conclusions: These data suggest IWAT lipolysis activates local IWAT afferents triggering a neural circuit from WAT to BAT that acutely induces BAT thermogenesis.
Keywords: Adipose innervation; BAT thermogenesis; Denervation; Lipolysis; WAT sensory.
Figures





References
-
- Youngstrom T.G., Bartness T.J. Catecholaminergic innervation of white adipose tissue in the Siberian hamster. American Journal of Physiology. 1995;268:R744–R751. - PubMed
-
- Giordano A. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: an immunohistochemical and ultrastructural investigation. Journal of Neurocytology. 1996;25:125–136. - PubMed
-
- Fishman R.B., Dark J. Sensory innervation of white adipose tissue. American Journal of Physiology. 1987;253:R942–R944. - PubMed
-
- Niijima A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. Journal of the Autonomic Nervous System. 1998;73(1):19–25. - PubMed
-
- Caro J.F. Leptin: the tale of an obesity gene. Diabetes. 1996;45(11):1455–1462. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

