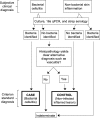
Toward an Objective Diagnostic Test for Bacterial Cellulitis
- PMID: 27656884
- PMCID: PMC5033594
- DOI: 10.1371/journal.pone.0162947
Toward an Objective Diagnostic Test for Bacterial Cellulitis
Abstract
Background: Prior studies repeatedly showed that cultures of skin lesions diagnosed as "cellulitis" are usually negative. However, lack of a gold standard for diagnosis (against which culture might be judged) and failure to assess the human immune response are important limitations of prior work. In this pilot study, we aimed to develop a criterion standard for research on bacterial cellulitis, to evaluate the sensitivity of procalcitonin for bacterial cellulitis, and to use gene expression analysis to find other candidate diagnostic markers.
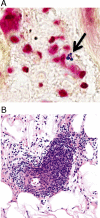
Methods: We classified lesions via biopsies, 16s rRNA gene detection, culture, and histopathology. We quantified procalcitonin expression in blood. We also used Nanostring technology to quantify transcription of immunomodulators that may distinguish cases from inflamed controls.
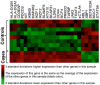
Results: Of 28 participants, 15 had a clinical diagnosis of cellulitis, six had a diagnosis of non-infectious dermatitis, and seven were normal volunteers. Of the "cellulitis" patients, three (20%) had pathogens isolated, and were designated confirmed cases. Procalcitonin was undetectable in all three. HLA-DQA1 was expressed 34-fold more in confirmed cases vs. controls (fold change of geometric mean). Heat maps depicting multiplex gene expression analysis revealed a distinct profile of gene expression in confirmed cases relative to comparators.
Conclusions: Most "cellulitis" patients had microbiologically-negative biopsies. Procalcitonin was undetectable, and HLA-DQA1 elevated, in confirmed bacterial cases. Multivariable transcriptomic profiling results supported our algorithm's ability to identify patients with true bacterial cellulitis. A larger sample may allow discovery of an immunological signature capable of distinguishing bacterial cellulitis from its mimics in clinical practice.
Conflict of interest statement
The authors received funding for this study from Biomerieux Inc., a commercial company and the manufacturer of procalcitonin assay utilized in the study. There are no other patents, products in development or marketed products to declare. This does not alter our adherence to all the PLOS ONE policies on sharing data and materials.
Figures


References
-
- Chambers HF (2013) Cellulitis, by any other name. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 56: 1763–1764. - PubMed
-
- Arakaki RY, Strazzula L, Woo E, Kroshinsky D (2014) The impact of dermatology consultation on diagnostic accuracy and antibiotic use among patients with suspected cellulitis seen at outpatient internal medicine offices: a randomized clinical trial. JAMA dermatology 150: 1056–1061. 10.1001/jamadermatol.2014.1085 - DOI - PubMed
-
- David CV, Chira S, Eells SJ, Ladrigan M, Papier A, et al. (2011) Diagnostic accuracy in patients admitted to hospitals with cellulitis. Dermatol Online J 17: 1. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

