The Dynamics of Platelet Activation during the Progression of Streptococcal Sepsis
- PMID: 27656898
- PMCID: PMC5033464
- DOI: 10.1371/journal.pone.0163531
The Dynamics of Platelet Activation during the Progression of Streptococcal Sepsis
Abstract
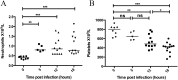
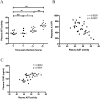
Platelets contribute to inflammation however, the role of platelet activation during the pathophysiological response to invasive bacterial infection and sepsis is not clear. Herein, we have investigated platelet activation in a mouse model of invasive Streptococcus pyogenes infection at 5, 12, and 18 hours post infection and correlated this to parameters of infection. The platelet population in ex-vivo blood samples showed no increased integrin activation or surface presentation of CD62P, however platelet-neutrophil complex formation and plasma levels of CD62P were increased during bacterial dissemination and the progression of sepsis, indicating that platelet activation had occurred in vivo. Platelet-neutrophil complex formation was the most discriminatory marker of platelet activation. Platelet-neutrophil complexes were increased above baseline levels during early sepsis but decreased to significantly lower levels than baseline during late sepsis. The removal of these complexes from the circulation coincided with a significant increase in organ damage and the accumulation of platelets in the liver sinusoids, suggesting that platelet activation in the circulation precedes accumulation of platelets in damaged organs. The results demonstrate that monitoring platelet activation using complementary methods may provide prognostic information during the pathogenesis of invasive S. pyogenes infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Platelets promote bacterial dissemination in a mouse model of streptococcal sepsis.Microbes Infect. 2013 Sep-Oct;15(10-11):669-76. doi: 10.1016/j.micinf.2013.05.003. Epub 2013 May 25. Microbes Infect. 2013. PMID: 23711899
-
Distinct Serotypes of Streptococcal M Proteins Mediate Fibrinogen-Dependent Platelet Activation and Proinflammatory Effects.Infect Immun. 2022 Feb 17;90(2):e0046221. doi: 10.1128/IAI.00462-21. Epub 2021 Dec 13. Infect Immun. 2022. PMID: 34898252 Free PMC article.
-
Complement Activation Occurs at the Surface of Platelets Activated by Streptococcal M1 Protein and This Results in Phagocytosis of Platelets.J Immunol. 2019 Jan 15;202(2):503-513. doi: 10.4049/jimmunol.1800897. Epub 2018 Dec 12. J Immunol. 2019. PMID: 30541884
-
Platelet-streptococcal interactions in endocarditis.Crit Rev Oral Biol Med. 1996;7(3):222-36. doi: 10.1177/10454411960070030201. Crit Rev Oral Biol Med. 1996. PMID: 8909879 Review.
-
Platelets and platelet extracellular vesicles in hemostasis and sepsis.J Investig Med. 2020 Apr;68(4):813-820. doi: 10.1136/jim-2019-001195. Epub 2019 Dec 15. J Investig Med. 2020. PMID: 31843956 Review.
Cited by
-
Neutrophil-Derived Extracellular Vesicles Activate Platelets after Pneumolysin Exposure.Cells. 2021 Dec 18;10(12):3581. doi: 10.3390/cells10123581. Cells. 2021. PMID: 34944089 Free PMC article.
-
Neutrophil Extracellular DNA Traps in Response to Infection or Inflammation, and the Roles of Platelet Interactions.Int J Mol Sci. 2024 Mar 5;25(5):3025. doi: 10.3390/ijms25053025. Int J Mol Sci. 2024. PMID: 38474270 Free PMC article. Review.
-
Platelet P2Y12 signalling pathway in the dysregulated immune response during sepsis.Br J Pharmacol. 2024 Feb;181(4):532-546. doi: 10.1111/bph.16207. Epub 2023 Aug 29. Br J Pharmacol. 2024. PMID: 37525937 Free PMC article. Review.
-
Platelets, Macrophages, and Thromboinflammation in Chagas Disease.J Inflamm Res. 2022 Oct 4;15:5689-5706. doi: 10.2147/JIR.S380896. eCollection 2022. J Inflamm Res. 2022. PMID: 36217453 Free PMC article. Review.
-
Role of matrix metalloproteinases 2 and 9, toll-like receptor 4 and platelet-leukocyte aggregate formation in sepsis-associated thrombocytopenia.PLoS One. 2018 May 7;13(5):e0196478. doi: 10.1371/journal.pone.0196478. eCollection 2018. PLoS One. 2018. PMID: 29734352 Free PMC article.
References
-
- Cohen J. The immunopathogenesis of sepsis. 2002;: 1–7. - PubMed
-
- Schouten M, Wiersinga WJ, Levi M, van der Poll T. Inflammation, endothelium, and coagulation in sepsis. J Leukoc Biol. 2007;83: 536–545. - PubMed
-
- Sharma B, Sharma A, Majumder M, Steier W, Sangal A, Kalawar M. Thrombocytopenia in septic shock patients—a prospective observational study of incidence, risk factors and correlation with clinical outcome. Anaesthesia and Intensive Care. 2008;35: 1–8. - PubMed
-
- Vandijck DM, Blot S, De Waele Jan J, Hoste EA, Vandewoude KH, Decruyenaere JM. Thrombocytopenia and outcome in critically ill patients with bloodstream infection. Heart and Lung The Journal of Acute and Critical Care. Elsevier Inc; 2010;39: 21–26. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

