Candida antarctica Lipase B Immobilized onto Chitin Conjugated with POSS® Compounds: Useful Tool for Rapeseed Oil Conversion
- PMID: 27657054
- PMCID: PMC5037846
- DOI: 10.3390/ijms17091581
Candida antarctica Lipase B Immobilized onto Chitin Conjugated with POSS® Compounds: Useful Tool for Rapeseed Oil Conversion
Abstract
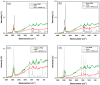
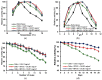
A new method is proposed for the production of a novel chitin-polyhedral oligomeric silsesquioxanes (POSS) enzyme support. Analysis by such techniques as X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy confirmed the effective functionalization of the chitin surface. The resulting hybrid carriers were used in the process of immobilization of the lipase type b from Candida antarctica (CALB). Fourier transform infrared spectroscopy (FTIR) confirmed the effective immobilization of the enzyme. The tests of the catalytic activity showed that the resulting support-biocatalyst systems remain hydrolytically active (retention of the hydrolytic activity up to 87% for the chitin + Methacryl POSS® cage mixture (MPOSS) + CALB after 24 h of the immobilization), as well as represents good thermal and operational stability, and retain over 80% of its activity in a wide range of temperatures (30-60 °C) and pH (6-9). Chitin-POSS-lipase systems were used in the transesterification processes of rapeseed oil at various reaction conditions. Produced systems allowed the total conversion of the oil to fatty acid methyl esters (FAME) and glycerol after 24 h of the process at pH 10 and a temperature 40 °C, while the Methacryl POSS® cage mixture (MPOSS) was used as a chitin-modifying agent.
Keywords: Candida antarctica lipase B; chitin; enzyme immobilization; polyhedral oligomeric silsesquioxanes; rapeseed oil transesterification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Immobilization of Candida antarctica Lipase B on Magnetic Poly(Urea-Urethane) Nanoparticles.Appl Biochem Biotechnol. 2016 Oct;180(3):558-575. doi: 10.1007/s12010-016-2116-6. Epub 2016 May 16. Appl Biochem Biotechnol. 2016. PMID: 27184256
-
Plasma Functionalized Multiwalled Carbon Nanotubes for Immobilization of Candida antarctica Lipase B: Production of Biodiesel from Methanolysis of Rapeseed Oil.Appl Biochem Biotechnol. 2016 Mar;178(5):974-89. doi: 10.1007/s12010-015-1922-6. Epub 2015 Nov 20. Appl Biochem Biotechnol. 2016. PMID: 26588921
-
A novel step towards immobilization of biocatalyst using agro waste and its application for ester synthesis.Int J Biol Macromol. 2018 Oct 1;117:366-376. doi: 10.1016/j.ijbiomac.2018.05.005. Epub 2018 May 4. Int J Biol Macromol. 2018. PMID: 29733931
-
Ethyl Butyrate Synthesis Catalyzed by Lipases A and B from Candida antarctica Immobilized onto Magnetic Nanoparticles. Improvement of Biocatalysts' Performance under Ultrasonic Irradiation.Int J Mol Sci. 2019 Nov 19;20(22):5807. doi: 10.3390/ijms20225807. Int J Mol Sci. 2019. PMID: 31752306 Free PMC article.
-
Solvothermal synthesis of hydrophobic chitin-polyhedral oligomeric silsesquioxane (POSS) nanocomposites.Int J Biol Macromol. 2015;78:224-9. doi: 10.1016/j.ijbiomac.2015.04.014. Epub 2015 Apr 15. Int J Biol Macromol. 2015. PMID: 25889055
Cited by
-
Pristine and Poly(Dimethylsiloxane) Modified Multi-Walled Carbon Nanotubes as Supports for Lipase Immobilization.Materials (Basel). 2021 May 27;14(11):2874. doi: 10.3390/ma14112874. Materials (Basel). 2021. PMID: 34072043 Free PMC article.
-
Desorption of Lipases Immobilized on Octyl-Agarose Beads and Coated with Ionic Polymers after Thermal Inactivation. Stronger Adsorption of Polymers/Unfolded Protein Composites.Molecules. 2017 Jan 5;22(1):91. doi: 10.3390/molecules22010091. Molecules. 2017. PMID: 28067789 Free PMC article.
-
New Strategy for the Immobilization of Lipases on Glyoxyl-Agarose Supports: Production of Robust Biocatalysts for Natural Oil Transformation.Int J Mol Sci. 2017 Oct 12;18(10):2130. doi: 10.3390/ijms18102130. Int J Mol Sci. 2017. PMID: 29023423 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

