MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma
- PMID: 27657493
- PMCID: PMC5470420
- DOI: 10.1001/jamaoncol.2016.3147
MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma
Abstract
Importance: Current recommendations for patients with cirrhosis are to undergo surveillance for hepatocellular carcinoma (HCC) with ultrasonography (US) every 6 months. However, the sensitivity of US screening to detect early-stage HCC is suboptimal. Magnetic resonance imaging (MRI) with liver-specific contrast may detect additional HCCs missed by US in high-risk patients with cirrhosis.
Objective: To compare the HCC detection rate of US and MRI in patients with cirrhosis who are at high risk for HCC.
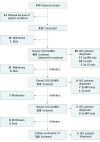
Design, setting, and participants: A prospective surveillance study of 407 patients with cirrhosis and an estimated annual risk of HCC greater than 5% who underwent 1 to 3 biannual screening examinations with paired US and liver-specific contrast-enhanced MRI at a tertiary care hospital between November 2011 and August 2014. All patients were followed-up with dynamic computed tomography (CT) at 6 months after the study. The confirmation of HCC was based on the results of histologic examination and/or typical CT images of HCC.
Main outcomes and measures: HCC detection rates and false-positive findings of US vs MRI.
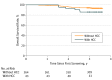
Results: A total of 407 eligible patients received 1100 screenings with paired US and MRI. Hepatocellular carcinomas were diagnosed in 43 patients: 1 detected by US only, 26 by MRI only, 11 by both, and 5 were missed by both. The HCC detection rate of MRI was 86.0% (37/43), significantly higher than the 27.9% (12/43) of US (P < .001). Magnetic resonance imaging showed a significantly lower rate of false-positive findings than US (3.0% vs 5.6%; P = .004). Of the 43 patients with HCC, 32 (74.4%) had very early-stage HCC (a single nodule <2 cm), and 29 (67.4%) received curative treatments. The 3-year survival rate of the patients with HCC (86.0%) was not inferior to those without HCC (94.2%; hazard ratio, 2.26; 95% CI, 0.92-5.56; P = .08).
Conclusions and relevance: In patients with cirrhosis at high-risk of HCC, screening that used MRI with liver-specific contrast resulted in a higher HCC detection rate and lower false-positive findings compared with US. With MRI screening, most of the cancers detected were at very early stage, which was associated with a high chance of curative treatments and favorable survival of patients. Whether surveillance with MRI would reduce mortality from HCC in high-risk patients requires further investigation.
Trial registration: clinicaltrials.gov Identifier: NCT01446666.
Conflict of interest statement
Figures
Comment in
-
Surveillance of Hepatocellular Carcinoma by Magnetic Resonance Imaging With Liver-Specific Contrast.JAMA Oncol. 2017 Apr 1;3(4):446-447. doi: 10.1001/jamaoncol.2016.3133. JAMA Oncol. 2017. PMID: 27656866 No abstract available.
Similar articles
-
Comparison of biannual ultrasonography and annual non-contrast liver magnetic resonance imaging as surveillance tools for hepatocellular carcinoma in patients with liver cirrhosis (MAGNUS-HCC): a study protocol.BMC Cancer. 2017 Dec 21;17(1):877. doi: 10.1186/s12885-017-3819-y. BMC Cancer. 2017. PMID: 29268722 Free PMC article.
-
Comparison of non-contrast abbreviated MRI and ultrasound as surveillance modalities for HCC.J Hepatol. 2024 Sep;81(3):461-470. doi: 10.1016/j.jhep.2024.03.048. Epub 2024 Apr 16. J Hepatol. 2024. PMID: 38636849
-
Noncontrast magnetic resonance imaging versus ultrasonography for hepatocellular carcinoma surveillance (MIRACLE-HCC): study protocol for a prospective randomized trial.BMC Cancer. 2018 Sep 24;18(1):915. doi: 10.1186/s12885-018-4827-2. BMC Cancer. 2018. PMID: 30249190 Free PMC article. Clinical Trial.
-
Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis.Gastroenterology. 2018 May;154(6):1706-1718.e1. doi: 10.1053/j.gastro.2018.01.064. Epub 2018 Feb 6. Gastroenterology. 2018. PMID: 29425931 Free PMC article.
-
Abbreviated MRI for Hepatocellular Carcinoma Screening and Surveillance.Radiographics. 2020 Nov-Dec;40(7):1916-1931. doi: 10.1148/rg.2020200104. Radiographics. 2020. PMID: 33136476 Free PMC article. Review.
Cited by
-
Gadoxetate-enhanced Abbreviated MRI for Hepatocellular Carcinoma Surveillance: Preliminary Experience.Radiol Imaging Cancer. 2019 Nov 29;1(2):e190010. doi: 10.1148/rycan.2019190010. eCollection 2019 Nov. Radiol Imaging Cancer. 2019. PMID: 33778680 Free PMC article.
-
Prediction of Decompensation and Death in Advanced Chronic Liver Disease Using Deep Learning Analysis of Gadoxetic Acid-Enhanced MRI.Korean J Radiol. 2022 Dec;23(12):1269-1280. doi: 10.3348/kjr.2022.0494. Korean J Radiol. 2022. PMID: 36447415 Free PMC article.
-
Evidence Supporting Diagnostic Value of Liver Imaging Reporting and Data System for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: A Systematic Review and Meta-analysis.J Biomed Phys Eng. 2024 Feb 1;14(1):5-20. doi: 10.31661/jbpe.v0i0.2211-1562. eCollection 2024 Feb. J Biomed Phys Eng. 2024. PMID: 38357604 Free PMC article. Review.
-
Hepatocellular carcinoma surveillance, incidence, and tumor doubling times in patients cured of hepatitis C.Cancer Med. 2022 May;11(9):1995-2005. doi: 10.1002/cam4.4508. Epub 2022 Mar 9. Cancer Med. 2022. PMID: 35261196 Free PMC article.
-
Utility of fusion imaging for the evaluation of ultrasound quality in hepatocellular carcinoma surveillance.Ultrasonography. 2023 Oct;42(4):580-588. doi: 10.14366/usg.23106. Epub 2023 Aug 15. Ultrasonography. 2023. PMID: 37722723 Free PMC article.
References
-
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127. - PubMed
-
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245-1255. - PubMed
-
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5)(suppl 1):S35-S50. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

