Rapid On-Site Evaluation Does Not Improve Endoscopic Ultrasound-Guided Fine Needle Aspiration Adequacy in Pancreatic Masses: A Meta-Analysis and Systematic Review
- PMID: 27657529
- PMCID: PMC5033338
- DOI: 10.1371/journal.pone.0163056
Rapid On-Site Evaluation Does Not Improve Endoscopic Ultrasound-Guided Fine Needle Aspiration Adequacy in Pancreatic Masses: A Meta-Analysis and Systematic Review
Abstract
Background and objectives: Rapid on-site evaluation (ROSE) during endoscopic ultrasonography-guided fine needle aspiration (EUS-FNA) of pancreatic masses has been reported to be associated with improved adequacy and diagnostic yield. However, recent observational data on the impact of ROSE have reported conflicting results. A meta-analysis and systematic review was therefore conducted to evaluate the contribution of ROSE during EUS-FNA of pancreatic masses.
Method: A systematic search was conducted in MEDLINE/Pubmed and EMBASE databases for studies comparing the efficacy of ROSE between patients in two cohorts. Outcomes considered included diagnostic adequate rate, diagnostic yield, number of needle passes, pooled sensitivity and specificity. Findings from a random-effects model were expressed as pooled risk difference (RD) with 95% confidence intervals (CIs).
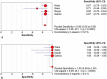
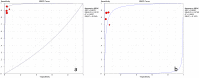
Results: A total of 7 studies (1299 patients) was finally included and further analyzed in the current meta-analysis. EUS-FNA with ROSE could not improve diagnostic adequacy (RD = 0.05, 95% CI: -0.01-0.11) and diagnostic yield (RD = 0.04 95%CI: -0.05, 0.13). The number of needle passes showed no statistically significant difference with and without ROSE (RD = -0.68 95%CI: -2.35, 0.98). The pooled sensitivity and specificity of ROSE group were 0.91 (95%CI: 0.87, 0.94) and 1 (95%CI: 0.94, 1.00). The pooled sensitivity and specificity of non-ROSE group were 0.85 (95%CI: 0.80, 0.89) and 1 (95%CI: 0.95, 1.00). ROSE group and non-ROSE group showed comparable sensitivity and specificity.
Conclusion: Compared to historical reports of its clinical efficacy in patients with pancreatic lesions, ROSE may be not associated with an improvement of diagnostic yield, adequate rate, pooled sensitivity and specificity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Erickson RA (2004) EUS-guided FNA. Gastrointest Endosc 60: 267–279. - PubMed
-
- Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM (1997) Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 112: 1087–1095. - PubMed
-
- Harewood GC, Wiersema MJ, Nelson H, Maccarty RL, Olson JE, Clain JE, et al. (2002) A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology 123: 24–32. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

