Diamagnetic Imaging Agents with a Modular Chemical Design for Quantitative Detection of β-Galactosidase and β-Glucuronidase Activities with CatalyCEST MRI
- PMID: 27657647
- PMCID: PMC6013409
- DOI: 10.1021/acs.bioconjchem.6b00482
Diamagnetic Imaging Agents with a Modular Chemical Design for Quantitative Detection of β-Galactosidase and β-Glucuronidase Activities with CatalyCEST MRI
Abstract
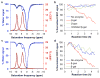
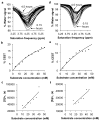
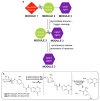
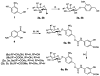
Imaging agents for the noninvasive in vivo detection of enzyme activity in preclinical and clinical settings could have fundamental implications in the field of drug discovery. Furthermore, a new class of targeted prodrug treatments takes advantage of high enzyme activity to tailor therapy and improve treatment outcomes. Herein, we report the design and synthesis of new magnetic resonance imaging (MRI) agents that quantitatively detect β-galactosidase and β-glucuronidase activities by measuring changes in chemical exchange saturation transfer (CEST). Based on a modular approach, we incorporated the enzymes' respective substrates to a salicylate moiety with a chromogenic spacer via a carbamate linkage. This furnished highly selective diamagnetic CEST agents that detected and quantified enzyme activities of glycoside hydrolase enzymes. Michaelis-Menten enzyme kinetics studies were performed by monitoring catalyCEST MRI signals, which were validated with UV-vis assays.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Simultaneous Evaluations of pH and Enzyme Activity with a CEST MRI Contrast Agent.ACS Sens. 2021 Dec 24;6(12):4535-4544. doi: 10.1021/acssensors.1c02408. Epub 2021 Dec 2. ACS Sens. 2021. PMID: 34856102 Free PMC article.
-
Assessments of tumor metabolism with CEST MRI.NMR Biomed. 2019 Oct;32(10):e3943. doi: 10.1002/nbm.3943. Epub 2018 Jun 25. NMR Biomed. 2019. PMID: 29938857 Free PMC article. Review.
-
Noninvasive detection of enzyme activity in tumor models of human ovarian cancer using catalyCEST MRI.Magn Reson Med. 2017 May;77(5):2005-2014. doi: 10.1002/mrm.26278. Epub 2016 May 25. Magn Reson Med. 2017. PMID: 27221386 Free PMC article.
-
A single diamagnetic catalyCEST MRI contrast agent that detects cathepsin B enzyme activity by using a ratio of two CEST signals.Contrast Media Mol Imaging. 2016 Mar-Apr;11(2):130-8. doi: 10.1002/cmmi.1672. Epub 2015 Dec 3. Contrast Media Mol Imaging. 2016. PMID: 26633584 Free PMC article.
-
Repurposing Clinical Agents for Chemical Exchange Saturation Transfer Magnetic Resonance Imaging: Current Status and Future Perspectives.Pharmaceuticals (Basel). 2020 Dec 24;14(1):11. doi: 10.3390/ph14010011. Pharmaceuticals (Basel). 2020. PMID: 33374213 Free PMC article. Review.
Cited by
-
Simultaneous Evaluations of pH and Enzyme Activity with a CEST MRI Contrast Agent.ACS Sens. 2021 Dec 24;6(12):4535-4544. doi: 10.1021/acssensors.1c02408. Epub 2021 Dec 2. ACS Sens. 2021. PMID: 34856102 Free PMC article.
-
In Situ Generated Novel 1H MRI Reporter for β-Galactosidase Activity Detection and Visualization in Living Tumor Cells.Front Chem. 2021 Jul 15;9:709581. doi: 10.3389/fchem.2021.709581. eCollection 2021. Front Chem. 2021. PMID: 34336792 Free PMC article.
-
Recent advances of sensing strategies for the detection of β-glucuronidase activity.Anal Bioanal Chem. 2022 Apr;414(9):2935-2951. doi: 10.1007/s00216-022-03921-y. Epub 2022 Mar 1. Anal Bioanal Chem. 2022. PMID: 35233695 Review.
-
Protein and peptide engineering for chemical exchange saturation transfer imaging in the age of synthetic biology.NMR Biomed. 2023 Jun;36(6):e4712. doi: 10.1002/nbm.4712. Epub 2022 Feb 27. NMR Biomed. 2023. PMID: 35150021 Free PMC article. Review.
-
Assessments of tumor metabolism with CEST MRI.NMR Biomed. 2019 Oct;32(10):e3943. doi: 10.1002/nbm.3943. Epub 2018 Jun 25. NMR Biomed. 2019. PMID: 29938857 Free PMC article. Review.
References
-
- Baruch A, Jeffery DA, Bogyo M. Enzyme activity – it’s all about image. Trends Cell Biol. 2004;14:29–35. - PubMed
-
- Glanemann C, Loos A, Gorret N, Willis LB, O’Brien XM, Lessard PA, Sinskey AJ. Disparity between changes in mRNA abundance and enzyme activity in Corynebacterium glutamicum: implications for DNA microarray analysis. Appl Microbiol Biotechnol. 2003;61:61–68. - PubMed
-
- de Graaf M, Boven E, Scheeren HW, Haisma HJ, Pinedo HM. Beta-glucuronidase mediated drug release. Curr Pharm Des. 2002;8:1391–1403. - PubMed
-
- Chen X, Wu B, Wang PG. Glucuronides in anti-cancer therapy. Curr Med Chem: Anti-Cancer Agents. 2003;3:139–150. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

