COMT Val158Met Polymorphism Modulates Huntington's Disease Progression
- PMID: 27657697
- PMCID: PMC5033325
- DOI: 10.1371/journal.pone.0161106
COMT Val158Met Polymorphism Modulates Huntington's Disease Progression
Abstract
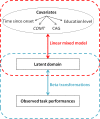
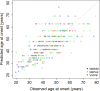
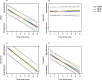
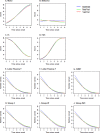
Little is known about the genetic factors modulating the progression of Huntington's disease (HD). Dopamine levels are affected in HD and modulate executive functions, the main cognitive disorder of HD. We investigated whether the Val158Met polymorphism of the catechol-O-methyltransferase (COMT) gene, which influences dopamine (DA) degradation, affects clinical progression in HD. We carried out a prospective longitudinal multicenter study from 1994 to 2011, on 438 HD gene carriers at different stages of the disease (34 pre-manifest; 172 stage 1; 130 stage 2; 80 stage 3; 17 stage 4; and 5 stage 5), according to Total Functional Capacity (TFC) score. We used the Unified Huntington's Disease Rating Scale to evaluate motor, cognitive, behavioral and functional decline. We genotyped participants for COMT polymorphism (107 Met-homozygous, 114 Val-homozygous and 217 heterozygous). 367 controls of similar ancestry were also genotyped. We compared clinical progression, on each domain, between groups of COMT polymorphisms, using latent-class mixed models accounting for disease duration and number of CAG (cytosine adenine guanine) repeats. We show that HD gene carriers with fewer CAG repeats and with the Val allele in COMT polymorphism displayed slower cognitive decline. The rate of cognitive decline was greater for Met/Met homozygotes, which displayed a better maintenance of cognitive capacity in earlier stages of the disease, but had a worse performance than Val allele carriers later on. COMT polymorphism did not significantly impact functional and behavioral performance. Since COMT polymorphism influences progression in HD, it could be used for stratification in future clinical trials. Moreover, DA treatments based on the specific COMT polymorphism and adapted according to disease duration could potentially slow HD progression.
Conflict of interest statement
R. de Diego-Balaguer, C. Schramm, I. Rebeix, E. Dupoux, A. Durr, A. Brice, P. Charles, L. Cleret de Langavant, K. Youssov, G. Fénelon, C. Verny, V. Damotte, JP Azulay, C. Simonin, C. Tranchant, P. Maison, A. Rialland, D. Schmitz, C. Jacquemot, B. Fontaine have nothing to disclose. A.C. Bachoud-Lévi acted as a consultant once for Teva in 2014 and reports grants from the National Reference Center for Huntington’s Disease from the Ministry of Health and from the clinical research directorate (AP-HP). C. Goizet reports grants from the European Huntington Disease Network and the Programme Hospitalier de Recherche Clinique (PHRC) during the duration of the study; personal fees came from Raptor Pharmaceutical France, Genzyme, and Actelion; grants from Genzyme, Association Française contre les myopathies (AFM), Association Strumpell-Lorrain (ASL), Connaître les Syndromes Cérébelleux (CSC), Union Nationale des Aveugles et Déficients Visuels (UNADEV), Conseil Régional d’Aquitaine (CRA), Agence Nationale pour la Recherche (ANR), Programme Hospitalier de Recherche Clinique (PHRC), Genzyme, Actelion, and Biomarin, not covering the work submitted here. This does not alter the authors' adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- The Huntington Collaborative Study Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72: 971–983. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

