Guidance Regarding Sample Collection and Refinement of Fecal Flotation Exam for the Isolation of Aspiculuris tetraptera
- PMID: 27657708
- PMCID: PMC5029824
Guidance Regarding Sample Collection and Refinement of Fecal Flotation Exam for the Isolation of Aspiculuris tetraptera
Abstract
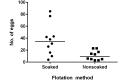
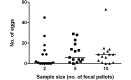
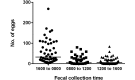
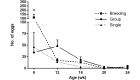
Aspiculuris tetraptera continues to be a problem in rodent vivaria, in part due to difficulties in parasite detection. Although PCR testing is highly sensitive, it is expensive and does not always provide immediate results. Consequently, many institutions rely on passive fecal flotation as a quick inhouse exam for diagnosing A. tetraptera infections. To increase the sensitivity of this test, we examined multiple parameters to determine the optimal test protocol. A 30-min soaking period prior to fecal flotation for 15 min allowed fecal pellets to soften and facilitated efficient egg isolation. We also evaluated the effect of time of day, sample size, age, sex, and housing status on egg isolation. No evidence of cyclical egg shedding was found, and although larger fecal sample sizes did not result in more eggs isolated, their use reduced the incidence of false-negative exams. The most eggs were isolated from 8- and 12-wk-old mice, and as mice aged, the number of eggs isolated declined. Overall, neither sex nor housing status influenced the number of eggs isolated. Finally, examination of multiple diagnostic tests (fecal flotation exam, direct examination of cecal and colonic contents, and fecal PCR) revealed that no single test was definitive, thus indicating that multiple tests might be required to successfully screen mice with low pinworm burdens. These findings provide guidance regarding sample selection, collection, and processing to efficiently detect A. tetraptera.
Figures



Similar articles
-
Evaluation of Traditional and Contemporary Methods for Detecting Syphacia obvelata and Aspiculuris tetraptera in Laboratory Mice.J Am Assoc Lab Anim Sci. 2017 Jan 1;56(1):32-41. J Am Assoc Lab Anim Sci. 2017. PMID: 28905712 Free PMC article.
-
False-positive results after environmental pinworm PCR testing due to Rhabditid nematodes in Corncob bedding.J Am Assoc Lab Anim Sci. 2014 Nov;53(6):717-24. J Am Assoc Lab Anim Sci. 2014. PMID: 25650980 Free PMC article.
-
Comparison of traditional and PCR methods during screening for and confirmation of Aspiculuris tetraptera in a mouse facility.J Am Assoc Lab Anim Sci. 2011 Nov;50(6):904-9. J Am Assoc Lab Anim Sci. 2011. PMID: 22330785 Free PMC article.
-
Pinworm infections in laboratory rodents: a review.Lab Anim. 1976 Jan;10(1):1-13. doi: 10.1258/002367776780948862. Lab Anim. 1976. PMID: 768631 Review.
-
American Association of Veterinary Parasitologists' review of veterinary fecal flotation methods and factors influencing their accuracy and use--is there really one best technique?Vet Parasitol. 2014 Jul 30;204(1-2):73-80. doi: 10.1016/j.vetpar.2014.05.009. Epub 2014 May 17. Vet Parasitol. 2014. PMID: 24893692 Review.
Cited by
-
PCR and RT-PCR in the Diagnosis of Laboratory Animal Infections and in Health Monitoring.J Am Assoc Lab Anim Sci. 2020 Sep 1;59(5):458-468. doi: 10.30802/AALAS-JAALAS-20-000008. Epub 2020 Jun 24. J Am Assoc Lab Anim Sci. 2020. PMID: 32580820 Free PMC article.
-
PCR Testing of IVC Filter Tops as a Method for Detecting Murine Pinworms and Fur Mites.J Am Assoc Lab Anim Sci. 2017 Nov 1;56(6):752-761. J Am Assoc Lab Anim Sci. 2017. PMID: 29256370 Free PMC article.
References
-
- Anya AO. 1966. Experimental studies on the physiology of hatching of Aspiculuris tetraptera Schulz (Oxyuridea:Nematoda). Parasitology 56:733–744. - PubMed
-
- Baker DG. 2007. Flynn's parasites of laboratory animals, 2nd ed. Ames (IA): Blackwell Publishing.
-
- Behnke JM. 1974. Proceedings: the effect of hydrocortisone upon infection with Aspiculuris tetraptera in laboratory mice. Parasitology 69:xviii. - PubMed
-
- Behnke JM. 1975. Immune expulsion of the nematode Aspiculuris tetraptera from mice given primary and challenge infections. Int J Parasitol 5:511–515. - PubMed
-
- Bunte R, Nolan R. 2006. Searching for Aspiculuris tetraptera: lessons learned. J Am Assoc Lab Anim Sci 45:86.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
