Tetracyclic indolines as a novel class of β-lactam-selective resistance-modifying agent for MRSA
- PMID: 27657810
- PMCID: PMC5148702
- DOI: 10.1016/j.ejmech.2016.09.034
Tetracyclic indolines as a novel class of β-lactam-selective resistance-modifying agent for MRSA
Abstract
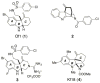
Antibiotic-resistant bacterial infections have seen a marked increase in recent years, while antibiotic discovery has waned. Resistance-modifying agents (RMA) offer an intriguing alternative strategy to fight against resistant bacteria. Here we report the discovery, antibiotic profiling, and structure-activity relationships of a novel class of RMAs, tetracyclic indolines. These selectively potentiate β-lactam antibiotics in methicillin-resistant Staphylococcus aureus (MRSA) without antibacterial or β-lactamase inhibitory activity on their own. The most potent analogue, 6a, showed strong potentiation of amoxicillin/clavulanic acid in a variety of hospital-acquired and community-acquired MRSA strains with low mammalian toxicity. These compounds may be further developed to extend the clinic life span of β-lactam antibiotics.
Keywords: Antibiotic resistance; Indoline alkaloids; Methicillin-resistant Staphylococcus aureus; Resistance-modifying agent; Structure-activity relationship; β-lactam.
Copyright © 2016 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
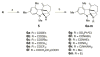

Similar articles
-
Bio-inspired synthesis yields a tricyclic indoline that selectively resensitizes methicillin-resistant Staphylococcus aureus (MRSA) to β-lactam antibiotics.Proc Natl Acad Sci U S A. 2013 Sep 24;110(39):15573-8. doi: 10.1073/pnas.1310459110. Epub 2013 Sep 9. Proc Natl Acad Sci U S A. 2013. PMID: 24019472 Free PMC article.
-
Gold-Catalyzed Cyclization Leads to a Bridged Tetracyclic Indolenine that Represses β-Lactam Resistance.Angew Chem Int Ed Engl. 2015 Aug 10;54(33):9546-9. doi: 10.1002/anie.201503736. Epub 2015 Jun 12. Angew Chem Int Ed Engl. 2015. PMID: 26074499
-
Restoring methicillin-resistant Staphylococcus aureus susceptibility to β-lactam antibiotics.Sci Transl Med. 2012 Mar 21;4(126):126ra35. doi: 10.1126/scitranslmed.3003592. Sci Transl Med. 2012. PMID: 22440737
-
Indole and Indoline Scaffolds in Antimicrobials: Overview, Synthesis and Recent Advances in Antimicrobial Research.Curr Med Chem. 2021;28(24):4828-4844. doi: 10.2174/0929867327666201102114923. Curr Med Chem. 2021. PMID: 33138747 Review.
-
Antimicrobial resistance: Not community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)! A clinician's guide to community MRSA - its evolving antimicrobial resistance and implications for therapy.Clin Infect Dis. 2011 Jan 1;52(1):99-114. doi: 10.1093/cid/ciq067. Clin Infect Dis. 2011. PMID: 21148528 Review.
Cited by
-
Zn(OTf)2-mediated annulations of N-propargylated tetrahydrocarbolines: divergent synthesis of four distinct alkaloidal scaffolds.Chem Sci. 2019 May 1;10(22):5686-5698. doi: 10.1039/c9sc01507h. eCollection 2019 Jun 14. Chem Sci. 2019. PMID: 31293753 Free PMC article.
-
Development and Application of Indolines in Pharmaceuticals.ChemistryOpen. 2023 Feb;12(2):e202200235. doi: 10.1002/open.202200235. ChemistryOpen. 2023. PMID: 36722823 Free PMC article. Review.
-
Resensitization of methicillin-resistant Staphylococcus aureus by amoxapine, an FDA-approved antidepressant.Heliyon. 2018 Jan 12;4(1):e00501. doi: 10.1016/j.heliyon.2017.e00501. eCollection 2018 Jan. Heliyon. 2018. PMID: 29349359 Free PMC article.
-
Enantioselective Tandem Cyclization of Alkyne-Tethered Indoles Using Cooperative Silver(I)/Chiral Phosphoric Acid Catalysis.Angew Chem Int Ed Engl. 2017 Sep 25;56(40):12206-12209. doi: 10.1002/anie.201706694. Epub 2017 Aug 23. Angew Chem Int Ed Engl. 2017. PMID: 28746772 Free PMC article.
-
Serendipitous Discovery of a Highly Active and Selective Resistance-Modifying Agent for Colistin-Resistant Gram-Negative Bacteria.ACS Omega. 2022 Mar 30;7(14):12442-12446. doi: 10.1021/acsomega.2c01530. eCollection 2022 Apr 12. ACS Omega. 2022. PMID: 35449921 Free PMC article.
References
-
- Fleming A. PA Norstedt & Söner. 1947. Nobel lecture on penicillin.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

