Dynamic Computational Model of Symptomatic Bacteremia to Inform Bacterial Separation Treatment Requirements
- PMID: 27657881
- PMCID: PMC5033423
- DOI: 10.1371/journal.pone.0163167
Dynamic Computational Model of Symptomatic Bacteremia to Inform Bacterial Separation Treatment Requirements
Abstract
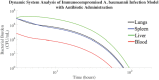
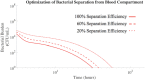
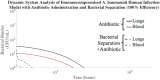
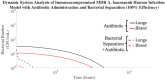
The rise of multi-drug resistance has decreased the effectiveness of antibiotics, which has led to increased mortality rates associated with symptomatic bacteremia, or bacterial sepsis. To combat decreasing antibiotic effectiveness, extracorporeal bacterial separation approaches have been proposed to capture and separate bacteria from blood. However, bacteremia is dynamic and involves host-pathogen interactions across various anatomical sites. We developed a mathematical model that quantitatively describes the kinetics of pathogenesis and progression of symptomatic bacteremia under various conditions, including bacterial separation therapy, to better understand disease mechanisms and quantitatively assess the biological impact of bacterial separation therapy. Model validity was tested against experimental data from published studies. This is the first multi-compartment model of symptomatic bacteremia in mammals that includes extracorporeal bacterial separation and antibiotic treatment, separately and in combination. The addition of an extracorporeal bacterial separation circuit reduced the predicted time of total bacteria clearance from the blood of an immunocompromised rodent by 49%, compared to antibiotic treatment alone. Implementation of bacterial separation therapy resulted in predicted multi-drug resistant bacterial clearance from the blood of a human in 97% less time than antibiotic treatment alone. The model also proposes a quantitative correlation between time-dependent bacterial load among tissues and bacteremia severity, analogous to the well-known 'area under the curve' for characterization of drug efficacy. The engineering-based mathematical model developed may be useful for informing the design of extracorporeal bacterial separation devices. This work enables the quantitative identification of the characteristics required of an extracorporeal bacteria separation device to provide biological benefit. These devices will potentially decrease the bacterial load in blood. Additionally, the devices may achieve bacterial separation rates that allow consequent acceleration of bacterial clearance in other tissues, inhibiting the progression of symptomatic bacteremia, including multi-drug resistant variations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Using the rate of bacterial clearance determined by real-time polymerase chain reaction as a timely surrogate marker to evaluate the appropriateness of antibiotic usage in critical patients with Acinetobacter baumannii bacteremia.Crit Care Med. 2012 Aug;40(8):2273-80. doi: 10.1097/CCM.0b013e3182515190. Crit Care Med. 2012. PMID: 22809902
-
Third generation cephalosporin resistant Enterobacteriaceae and multidrug resistant gram-negative bacteria causing bacteremia in febrile neutropenia adult cancer patients in Lebanon, broad spectrum antibiotics use as a major risk factor, and correlation with poor prognosis.Front Cell Infect Microbiol. 2015 Feb 12;5:11. doi: 10.3389/fcimb.2015.00011. eCollection 2015. Front Cell Infect Microbiol. 2015. PMID: 25729741 Free PMC article.
-
Resistance to empiric antimicrobial treatment predicts outcome in severe sepsis associated with Gram-negative bacteremia.J Hosp Med. 2011 Sep;6(7):405-10. doi: 10.1002/jhm.899. J Hosp Med. 2011. PMID: 21916003
-
Upflow anaerobic sludge blanket reactor--a review.Indian J Environ Health. 2001 Apr;43(2):1-82. Indian J Environ Health. 2001. PMID: 12397675 Review.
-
Peritonsillar abscess: clinical aspects of microbiology, risk factors, and the association with parapharyngeal abscess.Dan Med J. 2017 Mar;64(3):B5333. Dan Med J. 2017. PMID: 28260599 Review.
Cited by
-
A Computational Model of Bacterial Population Dynamics in Gastrointestinal Yersinia enterocolitica Infections in Mice.Biology (Basel). 2022 Feb 12;11(2):297. doi: 10.3390/biology11020297. Biology (Basel). 2022. PMID: 35205164 Free PMC article.
-
Immunoinformatics and Vaccine Development: An Overview.Immunotargets Ther. 2020 Feb 26;9:13-30. doi: 10.2147/ITT.S241064. eCollection 2020. Immunotargets Ther. 2020. PMID: 32161726 Free PMC article. Review.
References
-
- Wendt C, Grunwald WJ. Polyclonal bacteremia due to gram-negative rods. Clin Infect Dis. 2001;33(4):460–5. - PubMed
-
- Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–54. - PubMed
-
- Munford RS. Severe sepsis and septic shock: the role of gram-negative bacteremia. Annu Rev Pathol. 2006;1:467–96. - PubMed
-
- Poole K. Multidrug resistance in Gram-negative bacteria. Curr Opin Microbiol. 2001;4(5):500–8. - PubMed
-
- Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6(9):589–601. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

