Production Conditions Affect the In Vitro Anti-Tumoral Effects of a High Concentration Multi-Strain Probiotic Preparation
- PMID: 27657913
- PMCID: PMC5033378
- DOI: 10.1371/journal.pone.0163216
Production Conditions Affect the In Vitro Anti-Tumoral Effects of a High Concentration Multi-Strain Probiotic Preparation
Erratum in
-
Correction: Production Conditions Affect the In Vitro Anti-Tumoral Effects of a High Concentration Multi-Strain Probiotic Preparation.PLoS One. 2019 Feb 22;14(2):e0213134. doi: 10.1371/journal.pone.0213134. eCollection 2019. PLoS One. 2019. PMID: 30794716 Free PMC article.
Expression of concern in
-
Expression of Concern: Production Conditions Affect the In Vitro Anti-Tumoral Effects of a High Concentration Multi-Strain Probiotic Preparation.PLoS One. 2019 Feb 7;14(2):e0212403. doi: 10.1371/journal.pone.0212403. eCollection 2019. PLoS One. 2019. PMID: 30731003 Free PMC article. No abstract available.
Abstract
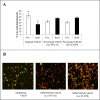
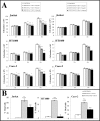
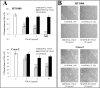
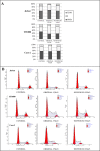
A careful selection of the probiotic agent, standardization of the dose and detailed characterization of the beneficial effects are essential when considering use of a probiotic for the dietary management of serious diseases. However, changes in the manufacturing processes, equipment or facilities can result in differences in the product itself due to the live nature of probiotics. The need to reconfirm safety and/or efficacy for any probiotic product made at a different factory is therefore mandatory. Recently, under the brand VSL#3®, a formulation produced by a manufacturer different from the previous one, has been commercialized in some European countries (the UK and Holland). VSL#3 is a high concentration multi-strain preparation which has been recognized by the main Gastroenterology Associations for the dietary management of pouchitis as well as ulcerative colitis. We have compared the "original" VSL#3 produced in USA with the "newfound" VSL#3 produced in Italy. According to our results, the "newfound" VSL#3 has 130-150% more "dead bacteria" compared to the "original" product, raising concerns for the well-known association between dead microbes with adverse effects. The abilities of bacterial lysates from the two formulations to influence in vitro viability and proliferation of different tumor cell lines also resulted different. The repair of previously scratched monolayers of various adherent tumor cell lines (i.e. HT1080, and Caco-2 cells) was inhibited more significantly by the "original" VSL#3 when compared to the "newfound" VSL#3. Tumor cell cycle profile, in particular cell cycle arrest and apoptotic death of the cancer cells, further confirms that the "original" VSL#3 has a better functional profile than the "newfound" VSL#3, at least in in vitro. Our data stress the importance of the production conditions for the "newfound" VSL#3 considering that this product is intended to be used for the dietary management of patients with very serious diseases, such as chronic inflammatory bowel diseases.
Conflict of interest statement
Dr. Van der Rest, the owner of BioVisible BV, has contributed to the present work as co-author. In particular, he provided his experience and expertise in the field of analysis related to the counts of bacteria. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

References
-
- FAO/WHO (2002) Guidelines for the Evaluation of Probiotics in Food. Working Group Report 2002. Rome and Geneva: Food and Agricultural Organization of the United Nations/World Health Organization.
-
- Safety of Probiotics to Reduce Risk and Prevent or Treat Disease. AHRQ Publication No. 11-E007 April 2011. Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services 540 Gaither Road Rockville, MD 20850 www.ahrq.gov
-
- Sanders ME, Klaenhammer TR, Ouwehand AC, et al. Effects of genetic, processing, or product formulation changes on efficacy and safety of probiotics. Annals of the New York Academy of Sciences 2014; 1309(1): 1–18. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

