Mitigation of indomethacin-induced gastrointestinal damages in fat-1 transgenic mice via gate-keeper action of ω-3-polyunsaturated fatty acids
- PMID: 27658533
- PMCID: PMC5034283
- DOI: 10.1038/srep33992
Mitigation of indomethacin-induced gastrointestinal damages in fat-1 transgenic mice via gate-keeper action of ω-3-polyunsaturated fatty acids
Abstract
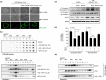
Non-steroidal anti-inflammatory drugs (NSAIDs) damage the gastrointestinal (GI) epithelial cell membranes by inducing several signals through lipid raft organization after membrane incorporation, whereas ω-3 polyunsaturated fatty acids (PUFAs) relieve inflammation, reduce oxidative stress, and provide cytoprotection, consequent to lipid raft disorganization. Therefore, we hypothesized that ω-3 PUFAs can protect the GI from NSAID-induced damages by initiating the gatekeeper action of cell membranes, subsequent to anti-inflammatory and anti-oxidative actions. Administration of indomethacin (IND) leads to the formation of lipid rafts and activation of caveolin-1; however, no such observations were made upon co-administration of eicosapentaenoic acid (EPA) and IND. In addition, the EPA-induced lipid raft disorganization, caveolin-1 inactivation, and cellular cytotoxicity were inhibited when target cells were knocked-out using G-protein coupled receptor 120 (GPR 120). EPA significantly attenuated IND-induced oxidative damage and apoptosis. IND administration induced significant ulceration, bleeding, and oedema in the stomach or small intestine of wild-type (WT) mice; however, such severe damages to the GI significantly decreased in fat-1 transgenic (TG) mice (P < 0.001), which exhibited decreased cyclooxygenase-2 expression and apoptosis, decreased interleukin-1β and FAS concentrations, and increased heme oxygenase-1 concentration. Our study indicates that the gatekeeper function of ω-3 PUFAs improves GI safety when administered with NSAID.
Figures




Similar articles
-
Omega-3 polyunsaturated fatty acids as an angelus custos to rescue patients from NSAID-induced gastroduodenal damage.J Gastroenterol. 2015 Jun;50(6):614-25. doi: 10.1007/s00535-014-1034-z. Epub 2015 Jan 13. J Gastroenterol. 2015. PMID: 25578017 Review.
-
S-allyl cysteine alleviates nonsteroidal anti-inflammatory drug-induced gastric mucosal damages by increasing cyclooxygenase-2 inhibition, heme oxygenase-1 induction, and histone deacetylation inhibition.J Gastroenterol Hepatol. 2014 Dec;29 Suppl 4:80-92. doi: 10.1111/jgh.12730. J Gastroenterol Hepatol. 2014. PMID: 25521739
-
Endogenous conversion of ω-6 to ω-3 polyunsaturated fatty acids in fat-1 mice attenuated intestinal polyposis by either inhibiting COX-2/β-catenin signaling or activating 15-PGDH/IL-18.Int J Cancer. 2016 May 1;138(9):2247-56. doi: 10.1002/ijc.29956. Epub 2015 Dec 31. Int J Cancer. 2016. PMID: 26650508
-
Overexpression and hyperactivity of breast cancer-associated fatty acid synthase (oncogenic antigen-519) is insensitive to normal arachidonic fatty acid-induced suppression in lipogenic tissues but it is selectively inhibited by tumoricidal alpha-linolenic and gamma-linolenic fatty acids: a novel mechanism by which dietary fat can alter mammary tumorigenesis.Int J Oncol. 2004 Jun;24(6):1369-83. Int J Oncol. 2004. PMID: 15138577
-
The effects of omega-3 polyunsaturated fatty acids on cardiac rhythm: a critical reassessment.Pharmacol Ther. 2013 Oct;140(1):53-80. doi: 10.1016/j.pharmthera.2013.05.011. Epub 2013 Jun 2. Pharmacol Ther. 2013. PMID: 23735203 Review.
Cited by
-
The Nonsteroidal Anti-Inflammatory Drug Ketorolac Alters the Small Intestinal Microbiota and Bile Acids Without Inducing Intestinal Damage or Delaying Peristalsis in the Rat.Front Pharmacol. 2021 Jun 4;12:664177. doi: 10.3389/fphar.2021.664177. eCollection 2021. Front Pharmacol. 2021. PMID: 34149417 Free PMC article.
-
Walnut polyphenol extracts inhibit Helicobacter pylori-induced STAT3Tyr705 phosphorylation through activation of PPAR-γ and SOCS1 induction.J Clin Biochem Nutr. 2020 Nov;67(3):248-256. doi: 10.3164/jcbn.20-89. Epub 2020 Sep 30. J Clin Biochem Nutr. 2020. PMID: 33293765 Free PMC article.
-
Heme Oxygenase-1 in Gastrointestinal Tract Health and Disease.Antioxidants (Basel). 2020 Dec 2;9(12):1214. doi: 10.3390/antiox9121214. Antioxidants (Basel). 2020. PMID: 33276470 Free PMC article. Review.
-
Plasma membrane integrity: implications for health and disease.BMC Biol. 2021 Apr 13;19(1):71. doi: 10.1186/s12915-021-00972-y. BMC Biol. 2021. PMID: 33849525 Free PMC article. Review.
-
Animal Models of Undernutrition and Enteropathy as Tools for Assessment of Nutritional Intervention.Nutrients. 2019 Sep 16;11(9):2233. doi: 10.3390/nu11092233. Nutrients. 2019. PMID: 31527523 Free PMC article. Review.
References
-
- Vane J. R. Purity and stability of synthetic peptides such as angiotensins and kinins. Nature 230, 382 (1971). - PubMed
-
- Wallace J. L. Nonsteroidal anti-inflammatory drugs and gastroenteropathy: the second hundred years. Gastroenterology 112, 1000–1016 (1997). - PubMed
-
- Giraud M. N., Motta C., Romero J. J., Bommelaer G. & Lichtenberger L. M. Interaction of indomethacin and naproxen with gastric surface-active phospholipids: a possible mechanism for the gastric toxicity of nonsteroidal anti-inflammatory drugs (NSAIDs). Biochemical pharmacology 57, 247–254 (1999). - PubMed
-
- Lichtenberger L. M. et al. Non-steroidal anti-inflammatory drugs (NSAIDs) associate with zwitterionic phospholipids: insight into the mechanism and reversal of NSAID-induced gastrointestinal injury. Nature medicine 1, 154–158 (1995). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

