Toll-Like Receptor 9 Activation Rescues Impaired Antibody Response in Needle-free Intradermal DNA Vaccination
- PMID: 27658623
- PMCID: PMC5034244
- DOI: 10.1038/srep33564
Toll-Like Receptor 9 Activation Rescues Impaired Antibody Response in Needle-free Intradermal DNA Vaccination
Abstract
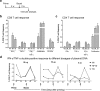
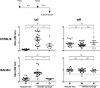
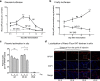
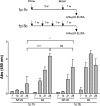
The delivery of plasmid DNA to the skin can target distinct subsets of dermal dendritic cells to confer a superior immune response. The needle-free immunization technology offers a reliable, safe and efficient means to administer intradermal (ID) injections. We report here that the ID injection of DNA vectors using an NF device (NF-ID) elicits a superior cell-mediated immune response, at much lesser DNA dosage, comparable in magnitude to the traditional intramuscular immunization. However, the humoral response is significantly impaired, possibly at the stage of B cell isotype switching. We found that the NF-ID administration deposits the DNA primarily on the epidermis resulting in a rapid loss of the DNA as well as the synthesized antigen due to the faster regeneration rate of the skin layers. Therefore, despite the immune-rich nature of the skin, the NF-ID immunization of DNA vectors may be limited by the impaired humoral response. Additional booster injections are required to augment the antibody response. As an alternative and a viable solution, we rescued the IgG response by coadministration of a Toll-like receptor 9 agonist, among other adjuvants examined. Our work has important implication for the optimization of the emerging needle-free technology for ID immunization.
Figures


References
-
- Pasparakis M., Haase I. & Nestle F. O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol 14, 289–301 (2014). - PubMed
-
- Di M. P., Perera G. K. & Nestle F. O. The multitasking organ: recent insights into skin immune function. Immunity 35, 857–869 (2011). - PubMed
-
- Malissen B., Tamoutounour S. & Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat. Rev. Immunol 14, 417–428 (2014). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

