Antibodies and superantibodies in patients with chronic rhinosinusitis with nasal polyps
- PMID: 27658758
- PMCID: PMC5380656
- DOI: 10.1016/j.jaci.2016.06.066
Antibodies and superantibodies in patients with chronic rhinosinusitis with nasal polyps
Abstract
Background: Chronic rhinosinusitis with nasal polyps is associated with local immunoglobulin hyperproduction and the presence of IgE antibodies against Staphylococcus aureus enterotoxins (SAEs). Aspirin-exacerbated respiratory disease is a severe form of chronic rhinosinusitis with nasal polyps in which nearly all patients express anti-SAEs.
Objectives: We aimed to understand antibodies reactive to SAEs and determine whether they recognize SAEs through their complementarity-determining regions (CDRs) or framework regions.
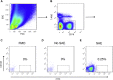
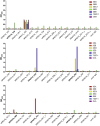
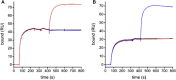
Methods: Labeled staphylococcal enterotoxin (SE) A, SED, and SEE were used to isolate single SAE-specific B cells from the nasal polyps of 3 patients with aspirin-exacerbated respiratory disease by using fluorescence-activated cell sorting. Recombinant antibodies with "matched" heavy and light chains were cloned as IgG1, and those of high affinity for specific SAEs, assayed by means of ELISA and surface plasmon resonance, were recloned as IgE and antigen-binding fragments. IgE activities were tested in basophil degranulation assays.
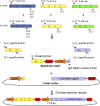
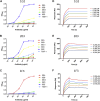
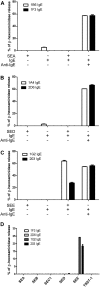
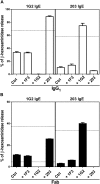
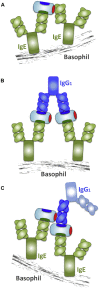
Results: Thirty-seven SAE-specific, IgG- or IgA-expressing B cells were isolated and yielded 6 anti-SAE clones, 2 each for SEA, SED, and SEE. Competition binding assays revealed that the anti-SEE antibodies recognize nonoverlapping epitopes in SEE. Unexpectedly, each anti-SEE mediated SEE-induced basophil degranulation, and IgG1 or antigen-binding fragments of each anti-SEE enhanced degranulation by the other anti-SEE.
Conclusions: SEEs can activate basophils by simultaneously binding as antigens in the conventional manner to CDRs and as superantigens to framework regions of anti-SEE IgE in anti-SEE IgE-FcεRI complexes. Anti-SEE IgG1s can enhance the activity of anti-SEE IgEs as conventional antibodies through CDRs or simultaneously as conventional antibodies and as "superantibodies" through CDRs and framework regions to SEEs in SEE-anti-SEE IgE-FcεRI complexes.
Keywords: Chronic rhinosinusitis with nasal polyps; Staphylococcus aureus enterotoxin; aspirin-exacerbated respiratory disease; basophil; superantibody; superantigen.
Copyright © 2016 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


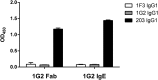

Similar articles
-
Role of local allergic inflammation and Staphylococcus aureus enterotoxins in Chinese patients with chronic rhinosinusitis with nasal polyps.J Laryngol Otol. 2017 Aug;131(8):707-713. doi: 10.1017/S0022215117001335. Epub 2017 Jul 7. J Laryngol Otol. 2017. PMID: 28683848
-
Superantigen-related TH2 CD4+ T cells in nonasthmatic chronic rhinosinusitis with nasal polyps.J Allergy Clin Immunol. 2020 May;145(5):1378-1388.e10. doi: 10.1016/j.jaci.2019.12.915. Epub 2020 Jan 25. J Allergy Clin Immunol. 2020. PMID: 31987845
-
Broad IgG repertoire in patients with chronic rhinosinusitis with nasal polyps regulates proinflammatory IgE responses.J Allergy Clin Immunol. 2019 Jun;143(6):2086-2094.e2. doi: 10.1016/j.jaci.2019.02.001. Epub 2019 Feb 11. J Allergy Clin Immunol. 2019. PMID: 30763592
-
Staphylococcus aureus enterotoxins as immune stimulants in chronic rhinosinusitis.Clin Allergy Immunol. 2007;20:163-75. Clin Allergy Immunol. 2007. PMID: 17534051 Review. No abstract available.
-
[New insights into the pathology of nasal polyposis: the role of superantigens and IgE].Verh K Acad Geneeskd Belg. 2005;67(1):5-28; discussion 29-32. Verh K Acad Geneeskd Belg. 2005. PMID: 15828304 Review. Dutch.
Cited by
-
Biomarkers in Chronic Rhinosinusitis with Nasal Polyps.Immunol Allergy Clin North Am. 2018 Nov;38(4):679-692. doi: 10.1016/j.iac.2018.06.006. Epub 2018 Sep 21. Immunol Allergy Clin North Am. 2018. PMID: 30342588 Free PMC article. Review.
-
Local Clonal Diversification and Dissemination of B Lymphocytes in the Human Bronchial Mucosa.Front Immunol. 2018 Sep 7;9:1976. doi: 10.3389/fimmu.2018.01976. eCollection 2018. Front Immunol. 2018. PMID: 30245687 Free PMC article.
-
Bacteriological analysis of selected phenotypes of chronic rhinosinusitis with co-existing asthma, allergy and hypersensitivity to non-steroidal anti-inflammatory drugs.Postepy Dermatol Alergol. 2021 Feb;38(2):57-62. doi: 10.5114/ada.2021.104279. Epub 2021 Mar 10. Postepy Dermatol Alergol. 2021. PMID: 34408567 Free PMC article.
-
Pathomechanisms of AERD-Recent Advances.Front Allergy. 2021 Sep 10;2:734733. doi: 10.3389/falgy.2021.734733. eCollection 2021. Front Allergy. 2021. PMID: 35387030 Free PMC article. Review.
-
Structure of a patient-derived antibody in complex with allergen reveals simultaneous conventional and superantigen-like recognition.Proc Natl Acad Sci U S A. 2018 Sep 11;115(37):E8707-E8716. doi: 10.1073/pnas.1806840115. Epub 2018 Aug 27. Proc Natl Acad Sci U S A. 2018. PMID: 30150373 Free PMC article.
References
-
- Huvenne W., Hellings P.W., Bachert C. Role of staphylococcal superantigens in airway disease. Int Arch Allergy Immunol. 2013;161:304–314. - PubMed
-
- Rossi R.E., Monasterolo G. Prevalence of serum IgE antibodies to the SAEs (SAE, SEB, SEC, SED, TSST-1) in patients with persistent allergic rhinitis. Int Arch Allergy Immunol. 2004;133:261–266. - PubMed
-
- Van Zele T., Gevaert P., Watelet J.B., Claeys G., Holtappels G., Claeys C. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114:981–983. - PubMed
-
- Bachert C., van Steen K., Zhang N., Holtappels G., Cattaert T., Maus B. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J Allergy Clin Immunol. 2012;130:376–381.e8. - PubMed
-
- Leung A.D., Schiltz A.M., Hall C.F., Liu A.H. Severe atopic dermatitis is associated with a high burden of environmental Staphylococcus aureus. Clin Exp Allergy. 2008;38:789–793. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

