Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: a stepped-wedge cluster-randomised trial
- PMID: 27658873
- PMCID: PMC5408866
- DOI: 10.1016/S2352-3018(16)30090-X
Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: a stepped-wedge cluster-randomised trial
Abstract
Background: In Africa, up to 30% of HIV-infected patients who are clinically eligible for antiretroviral therapy (ART) do not start timely treatment. We assessed the effects of an intervention targeting prevalent health systems barriers to ART initiation on timing and completeness of treatment initiation.
Methods: In this stepped-wedge, non-blinded, cluster-randomised controlled trial, 20 clinics in southwestern Uganda were randomly assigned in groups of five clinics every 6 months to the intervention by a computerised random number generator. This procedure continued until all clinics had crossed over from control (standard of care) to the intervention, which consisted of opinion-leader-led training and coaching of front-line health workers, a point-of-care CD4 cell count testing platform, a revised counselling approach without mandatory multiple pre-initiation sessions, and feedback to the facilities on their ART initiation rates and how they compared with other facilities. Treatment-naive, HIV-infected adults (aged ≥18 years) who were clinically eligible for ART during the study period were included in the study population. The primary outcome was ART initiation 14 days after first clinical eligibility for ART. This study is registered with ClinicalTrials.gov, number NCT01810289.
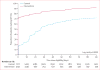
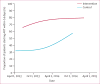
Findings: Between April 11, 2013, and Feb 2, 2015, 12 024 eligible patients visited one of the 20 participating clinics. Median CD4 count was 310 cells per μL (IQR 179-424). 3753 of 4747 patients (weighted proportion 80%) in the intervention group had started ART by 2 weeks after eligibility compared with 2585 of 7066 patients (38%) in the control group (risk difference 41·9%, 95% CI 40·1-43·8). Vital status was ascertained in a random sample of 208 patients in the intervention group and 199 patients in the control group. Four deaths (2%) occurred in the intervention group and five (3%) occurred in the control group.
Interpretation: A multicomponent intervention targeting health-care worker behaviour increased the probability of ART initiation 14 days after eligibility. This intervention consists of widely accessible components and has been tested in a real-world setting, and is therefore well positioned for use at scale.
Funding: National Institute of Allergy and Infectious Diseases (NIAID) and the President's Emergency Fund for AIDS Relief (PEPFAR).
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
We declare no competing interests.
Figures

Comment in
-
Accelerating initiation of antiretroviral therapy.Lancet HIV. 2016 Nov;3(11):e504-e505. doi: 10.1016/S2352-3018(16)30152-7. Epub 2016 Aug 27. Lancet HIV. 2016. PMID: 27658872 No abstract available.
References
-
- Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–94. - PubMed
-
- Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–29. - PubMed
-
- Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

