Characterizing the magnetic susceptibility tensor of lanthanide-containing polymethylated-DOTA complexes
- PMID: 27659040
- PMCID: PMC6628275
- DOI: 10.1007/s10858-016-0061-x
Characterizing the magnetic susceptibility tensor of lanthanide-containing polymethylated-DOTA complexes
Abstract
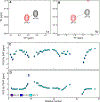
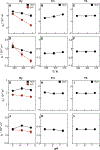
Lanthanide complexes based on the DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) cage are commonly used as phase contrast agents in magnetic resonance imaging, but can also be utilized in structural NMR applications due to their ability to induce either paramagnetic relaxation enhancement or a pseudocontact shift (PCS) depending on the choice of the lanthanide. The size and sign of the PCS for any given atom is determined by its coordinates relative to the metal center, and the characteristics of the lanthanide's magnetic susceptibility tensor. Using a polymethylated DOTA tag (Ln-M8-SPy) conjugated to ubiquitin, we calculated the position of the metal center and characterized the susceptibility tensor for a number of lanthanides (dysprosium, thulium, and ytterbium) under a range of pH and temperature conditions. We found that there was a difference in temperature sensitivity for each of the complexes studied, which depended on the size of the lanthanide ion as well as the isomeric state of the cage. Using 17O-NMR, we confirmed that the temperature sensitivity of the compounds was enhanced by the presence of an apically bound water molecule. Since amide-containing lanthanide complexes are known to be pH sensitive and can be used as probes of physiological pH, we also investigated the effect of pH on the Ln-M8-SPy susceptibility tensor, but we found that the changes in this pH range (5.0-7.4) were not significant.
Keywords: DOTA; Lanthanide; Magnetic susceptibility tensor; PCS; Pseudocontact shift.
Figures



References
-
- Aime S, Botta M, Ermondi G (1992) NMR study of solution structures and dynamics of lanthanide(III) complexes of DOTA. Inorg Chem 31:4291–4299. doi: 10.1021/ic00047a016 - DOI
-
- Aime S, Botta M, Ermondi G, Terreno E, Anelli PL, Fedeli F, Uggeri F (1996) Relaxometric, structural, and dynamic NMR studies of DOTA-like Ln(III) complexes (Ln = La, Gd, Ho, Yb) containing a p-nitrophenyl substituent. Inorg Chem 35:2726–2736. doi: 10.1021/ic950981u - DOI
-
- Aime S, Barge A, Botta M, Parker D, de Sousa AS (1997) Prototropic vs whole water exchange contributions to the solvent relaxation enhancement in the aqueous solution of a cationic Gd3+ macrocyclic complex. J Am Chem Soc 119:4767–4768. doi: 10.1021/ja963743m - DOI
-
- Aime S, Barge A, Botta M, De Sousa AS, Parker D (1998) Direct NMR spectroscopic observation of a lanthanide-coordinated water molecule whose exchange rate is dependent on the conformation of the complexes. Angew Chem Int Ed Engl 37:2673–2675. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2673:AID-ANIE2673>3.0.CO;2-# - DOI - PubMed
-
- Aime S et al. (1999) NMR, relaxometric, and structural studies of the hydration and exchange dynamics of cationic lanthanide complexes of macrocyclic tetraamide ligands. J Am Chem Soc 121:5762–5771. doi: 10.1021/ja990225d - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

