Nonvitamin K antagonist oral anticoagulant activity: challenges in measurement and reversal
- PMID: 27659071
- PMCID: PMC5034528
- DOI: 10.1186/s13054-016-1422-2
Nonvitamin K antagonist oral anticoagulant activity: challenges in measurement and reversal
Abstract
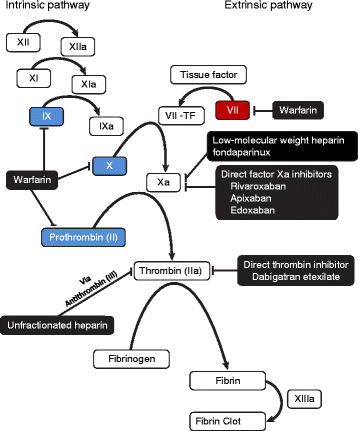
Background: Four nonvitamin K antagonist oral anticoagulants (NOACs) are approved for the prevention of stroke in patients with nonvalvular atrial fibrillation and for the treatment of venous thromboembolism. These include the direct thrombin inhibitor dabigatran and the direct factor Xa inhibitors rivaroxaban, apixaban, and edoxaban. Bleeding is a complication for all anticoagulants and concerns regarding bleeding risk and the suitability of effective reversal strategies may be a barrier to their prescription. Despite the reduced risk of bleeding compared with vitamin K antagonists, questions persist regarding the management of bleeding related to NOAC use.
Main text: To date, although a number of assays are responsive to NOACs, no single routine laboratory test has been identified to accurately measure the clinical anticoagulation state of patients on NOACs or established as a reliable predictor of bleeding risk. In addition, the establishment of a reliable human bleeding model to test novel inhibitors of the coagulation cascade has proved challenging. Although routine monitoring of anticoagulant levels is not necessary in patients taking NOACs, anticoagulant reversal and a means of measuring reversal may be required for patients who present with bleeding or require urgent surgery. Prothrombin complex concentrates are pooled plasma products containing varying amounts of inactive vitamin K-dependent clotting factors in addition to vitamin K-dependent proteins and can replenish factors in the intrinsic and extrinsic coagulation cascade, reversing an anticoagulant effect. Only one agent, idarucizumab, has been approved for rapid reversal of dabigatran-induced anticoagulation and one more agent, andexanet alfa, has been submitted for approval to reverse the anticoagulatory effects of direct and indirect factor Xa inhibitors.
Conclusions: This review discusses the laboratory tests available for assessing anticoagulation, human models of bleeding, and the use of current strategies-including prothrombin complex concentrates for reversal of anticoagulation by NOACs-to manage bleeding in patients.
Figures
References
-
- Pradaxa® (dabigatran etexilate mesylate) capsules for oral use. Full prescribing information. Ridgefield: Boehringer Ingelheim Pharmaceuticals, Inc.; 2015.
-
- Xarelto® (rivaroxaban) tablets for oral use. Full prescribing information. Titusville: Janssen Pharmaceuticals, Inc.; 2015.
-
- Eliquis® (apixaban) tablets for oral use. Full prescribing information. Princeton: Bristol-Myers Squibb Company; 2015.
-
- Savaysa™ (edoxaban) tablets for oral use. Full prescribing information. Parsippany: Daiichi Sankyo, Inc.; 2015.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

