Acute hyperglycemia suppresses left ventricular diastolic function and inhibits autophagic flux in mice under prohypertrophic stimulation
- PMID: 27659110
- PMCID: PMC5034479
- DOI: 10.1186/s12933-016-0452-z
Acute hyperglycemia suppresses left ventricular diastolic function and inhibits autophagic flux in mice under prohypertrophic stimulation
Abstract
Background: Left ventricular (LV) dysfunction is closely associated with LV hypertrophy or diabetes, as well as insufficient autophagic flux. Acute or chronic hyperglycemia is a prognostic factor for patients with myocardial infarction. However, the effect of acute hyperglycemia on LV dysfunction of the hypertrophic heart and the mechanisms involved are still unclear. This study aimed to confirm our hypothesis that either acute or chronic hyperglycemia suppresses LV diastolic function and autophagic flux.
Methods: The transverse aortic constriction (TAC) model and streptozocin-induced type 1 diabetic mellitus mice were used. LV function was evaluated with a Millar catheter. Autophagic levels and autophagic flux in the whole heart and cultured neonatal rat cardiomyocytes in response to hyperglycemia were examined by using western blotting of LC3B-II and P62. We also examined the effect of an autophagic inhibitor on LC3B-II and P62 protein expression and LC3 puncta.
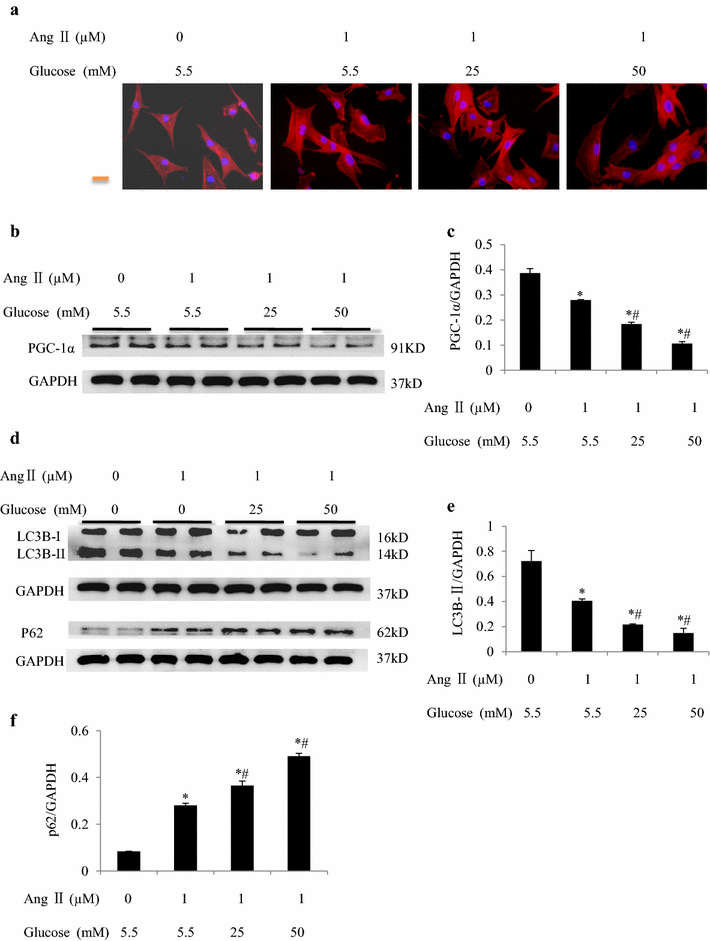
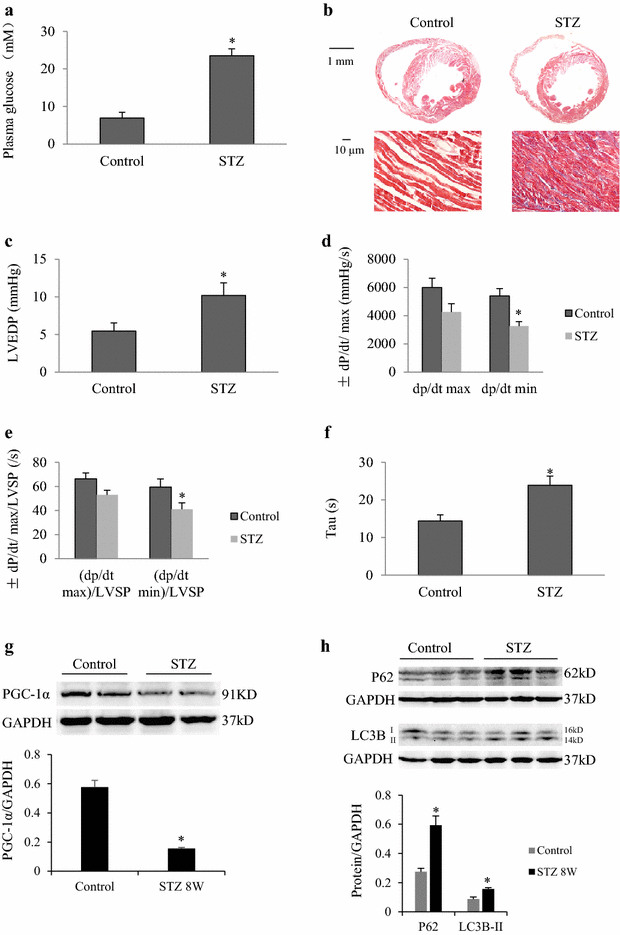
Results: In mice with TAC, we detected diastolic dysfunction as early as 30 min after TAC. This dysfunction was indicated by a greater LV end-diastolic pressure and the exponential time constant of LV relaxation, as well as a smaller maximum descending rate of LV pressure in comparison with sham group. Similar results were also obtained in mice with TAC for 2 weeks, in addition to increased insulin resistance. Acute hyperglycemic stress suppressed diastolic function in mice with myocardial hypertrophy, as evaluated by invasive LV hemodynamic monitoring. Mice with chronic hyperglycemia induced by streptozocin showed myocardial fibrosis and diastolic dysfunction. In high glucose-treated cardiomyocytes and streptozocin-treated mice, peroxisome proliferator-activated receptor-γ coactivator 1α was downregulated, while P62 was upregulated. Autophagic flux was also significantly inhibited in response to high glucose exposure in angiotensin-II treated cardiomyocytes.
Conclusions: Acute hyperglycemia suppresses diastolic function, damages mitochondrial energy signaling, and inhibits autophagic flux in prohypertrophic factor-stimulated cardiomyocytes.
Keywords: Acute hyperglycemia; Autophagic flux; Diastolic function; Myocardial hypertrophy.
Figures






 inhibition
inhibitionSimilar articles
-
[Autophagic flux of cardiomyocytes from 20-week transverse abdominal aortic constriction rats].Sheng Li Xue Bao. 2013 Jun 25;65(3):301-8. Sheng Li Xue Bao. 2013. PMID: 23788187 Chinese.
-
MMP9 inhibition increases autophagic flux in chronic heart failure.Am J Physiol Heart Circ Physiol. 2020 Dec 1;319(6):H1414-H1437. doi: 10.1152/ajpheart.00032.2020. Epub 2020 Oct 16. Am J Physiol Heart Circ Physiol. 2020. PMID: 33064567 Free PMC article.
-
Long non-coding RNA nuclear-enriched abundant transcript 1 inhibition blunts myocardial ischemia reperfusion injury via autophagic flux arrest and apoptosis in streptozotocin-induced diabetic rats.Atherosclerosis. 2018 Oct;277:113-122. doi: 10.1016/j.atherosclerosis.2018.08.031. Epub 2018 Aug 27. Atherosclerosis. 2018. PMID: 30205319
-
Clinical aspects of left ventricular diastolic function assessed by Doppler echocardiography following acute myocardial infarction.Dan Med Bull. 2001 Nov;48(4):199-210. Dan Med Bull. 2001. PMID: 11767125 Review.
-
Diastolic function in various forms of left ventricular hypertrophy: contribution of active Doppler stress echo.Int J Sports Med. 1996 Nov;17 Suppl 3:S184-90. doi: 10.1055/s-2007-972922. Int J Sports Med. 1996. PMID: 9119541 Review.
Cited by
-
Additive effect of admission hyperglycemia on left ventricular stiffness in patients following acute myocardial infarction verified by CMR tissue tracking.Cardiovasc Diabetol. 2024 Jun 20;23(1):210. doi: 10.1186/s12933-024-02295-y. Cardiovasc Diabetol. 2024. PMID: 38902730 Free PMC article.
-
Impact of acute hyperglycemia on layer-specific left ventricular strain in asymptomatic diabetic patients: an analysis based on two-dimensional speckle tracking echocardiography.Cardiovasc Diabetol. 2019 Jun 3;18(1):68. doi: 10.1186/s12933-019-0876-3. Cardiovasc Diabetol. 2019. PMID: 31159858 Free PMC article.
-
Hydrogen Sulfide Inhibits High Glucose-Induced Neuronal Senescence by Improving Autophagic Flux via Up-regulation of SIRT1.Front Mol Neurosci. 2019 Aug 20;12:194. doi: 10.3389/fnmol.2019.00194. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31481873 Free PMC article.
-
Impact of glycemic control on aortic stiffness, left ventricular mass and diastolic longitudinal function in type 2 diabetes mellitus.Cardiovasc Diabetol. 2017 Jun 17;16(1):78. doi: 10.1186/s12933-017-0557-z. Cardiovasc Diabetol. 2017. PMID: 28623932 Free PMC article.
-
Antihypertrophic Memory After Regression of Exercise-Induced Physiological Myocardial Hypertrophy Is Mediated by the Long Noncoding RNA Mhrt779.Circulation. 2021 Jun 8;143(23):2277-2292. doi: 10.1161/CIRCULATIONAHA.120.047000. Epub 2021 Mar 24. Circulation. 2021. PMID: 33757294 Free PMC article.
References
-
- Wei X, Wu B, Zhao J, Zeng Z, Xuan W, Cao S, Huang X, Asakura M, Xu D, Bin J, et al. Myocardial hypertrophic preconditioning attenuates cardiomyocyte hypertrophy and slows progression to heart failure through upregulation of S100A8/A9. Circulation. 2015;131(17):1506–1517. doi: 10.1161/CIRCULATIONAHA.114.013789. - DOI - PMC - PubMed
-
- Liao Y, Takashima S, Zhao H, Asano Y, Shintani Y, Minamino T, Kim J, Fujita M, Hori M, Kitakaze M. Control of plasma glucose with alpha-glucosidase inhibitor attenuates oxidative stress and slows the progression of heart failure in mice. Cardiovasc Res. 2006;70(1):107–116. doi: 10.1016/j.cardiores.2006.01.021. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

