Evidence-based selection of training compounds for use in the mechanism-based integrated prediction of drug-induced liver injury in man
- PMID: 27659300
- PMCID: PMC5104805
- DOI: 10.1007/s00204-016-1845-1
Evidence-based selection of training compounds for use in the mechanism-based integrated prediction of drug-induced liver injury in man
Abstract
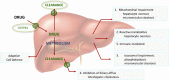
The current test systems employed by pharmaceutical industry are poorly predictive for drug-induced liver injury (DILI). The 'MIP-DILI' project addresses this situation by the development of innovative preclinical test systems which are both mechanism-based and of physiological, pharmacological and pathological relevance to DILI in humans. An iterative, tiered approach with respect to test compounds, test systems, bioanalysis and systems analysis is adopted to evaluate existing models and develop new models that can provide validated test systems with respect to the prediction of specific forms of DILI and further elucidation of mechanisms. An essential component of this effort is the choice of compound training set that will be used to inform refinement and/or development of new model systems that allow prediction based on knowledge of mechanisms, in a tiered fashion. In this review, we focus on the selection of MIP-DILI training compounds for mechanism-based evaluation of non-clinical prediction of DILI. The selected compounds address both hepatocellular and cholestatic DILI patterns in man, covering a broad range of pharmacologies and chemistries, and taking into account available data on potential DILI mechanisms (e.g. mitochondrial injury, reactive metabolites, biliary transport inhibition, and immune responses). Known mechanisms by which these compounds are believed to cause liver injury have been described, where many if not all drugs in this review appear to exhibit multiple toxicological mechanisms. Thus, the training compounds selection offered a valuable tool to profile DILI mechanisms and to interrogate existing and novel in vitro systems for the prediction of human DILI.
Keywords: DILI mechanisms; Drug-induced liver injury (DILI); Evidence-based selection; MIP-DILI; Set of training compounds.
Figures
Similar articles
-
Current limitations and future opportunities for prediction of DILI from in vitro.Arch Toxicol. 2017 Jan;91(1):131-142. doi: 10.1007/s00204-016-1874-9. Epub 2016 Oct 20. Arch Toxicol. 2017. PMID: 27766365 Review.
-
Metabolic activation and drug-induced liver injury: in vitro approaches for the safety risk assessment of new drugs.J Appl Toxicol. 2016 Jun;36(6):752-68. doi: 10.1002/jat.3277. Epub 2015 Dec 22. J Appl Toxicol. 2016. PMID: 26691983 Review.
-
Utilization of causal reasoning of hepatic gene expression in rats to identify molecular pathways of idiosyncratic drug-induced liver injury.Toxicol Sci. 2014 Jan;137(1):234-48. doi: 10.1093/toxsci/kft232. Epub 2013 Oct 17. Toxicol Sci. 2014. PMID: 24136188
-
Assessment of reactive metabolites in drug-induced liver injury.Arch Pharm Res. 2011 Nov;34(11):1879-86. doi: 10.1007/s12272-011-1108-x. Epub 2011 Dec 3. Arch Pharm Res. 2011. PMID: 22139687 Review.
-
Drug-induced liver injury.Handb Exp Pharmacol. 2010;(196):3-27. doi: 10.1007/978-3-642-00663-0_1. Handb Exp Pharmacol. 2010. PMID: 20020257 Review.
Cited by
-
Oxidative-stress and long-term hepatotoxicity: comparative study in Upcyte human hepatocytes and hepaRG cells.Arch Toxicol. 2022 Apr;96(4):1021-1037. doi: 10.1007/s00204-022-03236-y. Epub 2022 Feb 14. Arch Toxicol. 2022. PMID: 35156134
-
Comparison of Hepatic 2D Sandwich Cultures and 3D Spheroids for Long-term Toxicity Applications: A Multicenter Study.Toxicol Sci. 2018 Apr 1;162(2):655-666. doi: 10.1093/toxsci/kfx289. Toxicol Sci. 2018. PMID: 29329425 Free PMC article.
-
High-Content Screening for the Detection of Drug-Induced Oxidative Stress in Liver Cells.Antioxidants (Basel). 2021 Jan 13;10(1):106. doi: 10.3390/antiox10010106. Antioxidants (Basel). 2021. PMID: 33451093 Free PMC article. Review.
-
Drug Metabolism of Hepatocyte-like Organoids and Their Applicability in In Vitro Toxicity Testing.Molecules. 2023 Jan 7;28(2):621. doi: 10.3390/molecules28020621. Molecules. 2023. PMID: 36677681 Free PMC article.
-
Pre-clinical 2D and 3D toxicity response to a panel of nanomaterials; comparative assessment of NBM-induced liver toxicity.Drug Deliv Transl Res. 2022 Sep;12(9):2157-2177. doi: 10.1007/s13346-022-01170-1. Epub 2022 Jun 28. Drug Deliv Transl Res. 2022. PMID: 35763196 Free PMC article.
References
-
- Abboud G, Kaplowitz N. Drug-induced liver injury. Drug Saf. 2007;30:277–294. - PubMed
-
- Albers GW, Diener H-C, Frison L, et al. Ximelagatran vs warfarin for stroke prevention in patients with nonvalvular atrial fibrillation: a randomized trial. JAMA. 2005;293:690–698. - PubMed
-
- Ashrafian H, Horowitz JD, Frenneaux MP. Perhexiline. Cardiovasc Drug Rev. 2007;25:76–97. - PubMed
-
- Atiq M, Davis JC, Lamps LW, et al. Amiodarone induced liver cirrhosis. Report of two cases. J Gastrointest Liver Dis. 2009;18:233–235. - PubMed
-
- Babich MM, Pike I, Shiffman ML. Metformin-induced acute hepatitis. Am J Med. 1998;104:490–492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical