Human cathelicidin LL-37 enhance the antibiofilm effect of EGCG on Streptococcus mutans
- PMID: 27659310
- PMCID: PMC5034579
- DOI: 10.1186/s12903-016-0292-y
Human cathelicidin LL-37 enhance the antibiofilm effect of EGCG on Streptococcus mutans
Retraction in
-
Retraction Note: Human cathelicidin LL-37 enhance the antibiofilm effect of EGCG on Streptococcus mutans.BMC Oral Health. 2017 Jun 26;17(1):102. doi: 10.1186/s12903-017-0392-3. BMC Oral Health. 2017. PMID: 28651563 Free PMC article. No abstract available.
Abstract
Background: Streptococcus mutans forms biofilms as a resistance mechanism against antimicrobial agents in the human oral cavity. We recently showed that human cathelicidin LL-37 exhibits inhibitory effects on biofilm formation of S. mutans through interaction with lipoteichoic acid (LTA), but without antibacterial or biofilm dispersal abilities. (-)-Epigallocatechin gallate (EGCG) is the most abundant constituent of tea catechins that has the greatest anti-infective potential to inhibit the growth of various microorganisms and biofilm formation. Therefore, in this study, we evaluated whether LL-37 interacts with EGCG to enhance the antibiofilm effect of EGCG on S. mutans biofilm formation.
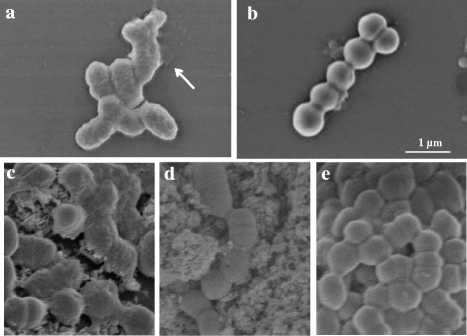
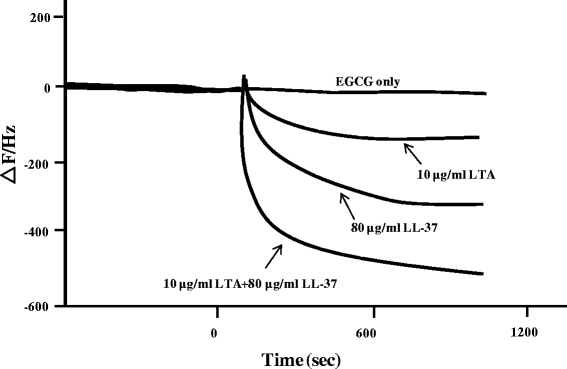
Methods: Clinical S. mutans strains (n = 10) isolated from children's saliva were tested in a biofilm formation assay. The antibiofilm effect of EGCG with and without LL-37 was analyzed by the minimum biofilm eradication concentration assay and confirmed using field emission-scanning electron microscopy. In addition, the interaction among EGCG, LL-37, and LTA of S. mutans was determined using quartz crystal microbalance analysis.
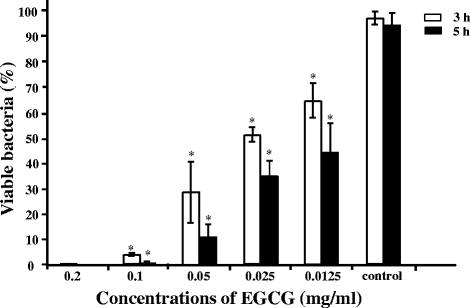
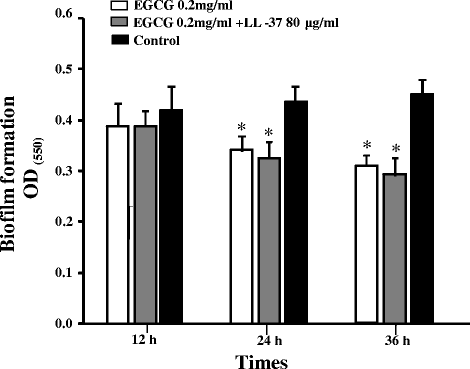
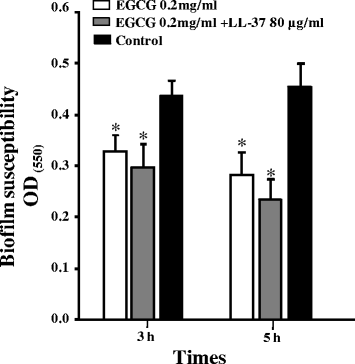
Results: EGCG killed 100 % of planktonic S. mutans within 5 h, inhibited biofilm formation within 24 h, and reduced bacteria cells in preformed biofilms within 3 h at a concentration of 0.2 mg/mL. However, EGCG did not appear to interact with LTA. LL-37 effectively enhanced the bactericidal activity of EGCG against biofilm formation and preformed biofilms as determined by quantitative crystal violet staining and field emission-scanning electron microscopy. In addition, quartz crystal microbalance analysis revealed that LL-37 interacted with EGCG and promoted binding between EGCG and LTA of S. mutans.
Conclusions: We show that LL-37 enhances the antibiofilm effect of EGCG on S. mutans. This finding provides new knowledge for dental treatment by using LL-37 as a potential antibiofilm compound.
Keywords: Biofilm; EGCG; LL-37; Streptococcus mutans.
Figures
Similar articles
-
Inhibitory effects of tea catechin epigallocatechin-3-gallate against biofilms formed from Streptococcus mutans and a probiotic lactobacillus strain.Arch Oral Biol. 2018 Oct;94:69-77. doi: 10.1016/j.archoralbio.2018.06.019. Epub 2018 Jun 26. Arch Oral Biol. 2018. PMID: 29979975
-
The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans.Antimicrob Agents Chemother. 2011 Mar;55(3):1229-36. doi: 10.1128/AAC.01016-10. Epub 2010 Dec 13. Antimicrob Agents Chemother. 2011. PMID: 21149622 Free PMC article.
-
Antimicrobial activity of tea catechin against canine oral bacteria and the functional mechanisms.J Vet Med Sci. 2016 Oct 1;78(9):1439-1445. doi: 10.1292/jvms.16-0198. Epub 2016 May 28. J Vet Med Sci. 2016. PMID: 27246281 Free PMC article.
-
Effects of the green tea catechin epigallocatechin-3-gallate on Streptococcus mutans planktonic cultures and biofilms: systematic literature review of in vitro studies.Biofouling. 2022 Aug;38(7):687-695. doi: 10.1080/08927014.2022.2116320. Epub 2022 Aug 26. Biofouling. 2022. PMID: 36017657
-
Antibiofilm properties of cathelicidin LL-37: an in-depth review.World J Microbiol Biotechnol. 2023 Feb 14;39(4):99. doi: 10.1007/s11274-023-03545-z. World J Microbiol Biotechnol. 2023. PMID: 36781570 Review.
Cited by
-
Regulation of LL-37 in Bone and Periodontium Regeneration.Life (Basel). 2022 Sep 30;12(10):1533. doi: 10.3390/life12101533. Life (Basel). 2022. PMID: 36294968 Free PMC article. Review.
-
Significance and Diagnostic Role of Antimicrobial Cathelicidins (LL-37) Peptides in Oral Health.Biomolecules. 2017 Dec 5;7(4):80. doi: 10.3390/biom7040080. Biomolecules. 2017. PMID: 29206168 Free PMC article. Review.
-
Retraction Note: Human cathelicidin LL-37 enhance the antibiofilm effect of EGCG on Streptococcus mutans.BMC Oral Health. 2017 Jun 26;17(1):102. doi: 10.1186/s12903-017-0392-3. BMC Oral Health. 2017. PMID: 28651563 Free PMC article. No abstract available.
-
Effect of pH-sensitive nanoparticles on inhibiting oral biofilms.Drug Deliv. 2022 Dec;29(1):561-573. doi: 10.1080/10717544.2022.2037788. Drug Deliv. 2022. PMID: 35156501 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

