Population Pharmacokinetic Analysis of Piperacillin/Tazobactam in Korean Patients with Acute Infections
- PMID: 27659435
- PMCID: PMC5048002
- DOI: 10.3947/ic.2016.48.3.209
Population Pharmacokinetic Analysis of Piperacillin/Tazobactam in Korean Patients with Acute Infections
Abstract
Background: For more effective and safer usage of antibiotics, the dosing strategy should be individualized based on the patients' characteristics, including race. The aim of this study was to investigate the population pharmacokinetic (PK) profiles of piperacillin and tazobactam in Korean patients with acute infections.
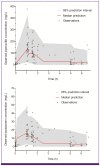
Materials and methods: At least four consecutive 2/0.25 g or 4/0.5 g doses of piperacillin/tazobactam (TZP) were intravenously infused over 1 h every 8 h for patients with creatinine clearance (CL(cr)) ≤50 ml/min or CL(cr) >50 mL/min, respectively. Blood samples from 33 patients at a steady-state were taken pre-dose and at 0 min, 30 min, and 4-6 h after the fourth infusion. The population PK analysis was conducted using a non-linear mixed-effects method. A likelihood ratio test was used to select significant covariates, with significance levels of P < 0.05 for selection and P < 0.01 for elimination.
Results: Both piperacillin PK and tazobactam PK were well described by a two-compartment model with first-order elimination. Creatinine clearance and body weight, as covariates on clearance (CL) and volume of central compartment (V1), were selected among the covariates possibly affecting PK parameters of both drugs. CL was defined as CL = 2.9 + 4.03 × CL(cr) /47 for piperacillin and CL = 1.76 + 4.81 × CL(cr) /47 for tazobactam. V1 was defined as V1 = 19.5 × weight/60 for piperacillin and V1 = 22.6 × weight/60 for tazobactam.
Conclusion: The PK profiles of TZP at a steady-state in Korean patients with acute infections were well described by a two-compartment model with first-order elimination. Both piperacillin and tazobactam clearances were significantly influenced by creatinine clearance.
Keywords: Clearance; Piperacillin; Population pharmacokinetics; Race; Tazobactam.
Conflict of interest statement
The study drug was provided by the Kuhnil Pharmaceutical Company.
Figures

References
-
- Gin A, Dilay L, Karlowsky JA, Walkty A, Rubinstein E, Zhanel GG. Piperacilin-tazobactam: a beta-lactam/beta-lactamase inhibitor combination. Expert Rev Anti Infect Ther. 2007;5:365–383. - PubMed
-
- Maltezou HC, Nikolaidis P, Lebesii E, Dimitriou L, Androulakakis E, Kafetzis DA. Piperacillin/tazobactam versus cefotaxime plus metronidazole for treatment of children with intra-abdominal infections requiring surgery. Eur J Clin Microbiol Infect Dis. 2001;20:643–646. - PubMed
-
- Tumbarello M, Sanguinetti M, Montuori E, Trecarichi EM, Posteraro B, Fiori B, Citton R, D’Inzeo T, Fadda G, Cauda R, Spanu T. Predictors of mortality in patients with bloodstream infections caused by extended-spectrum-beta-lactamase-producing Enterobacteriaceae: importance of inadequate initial antimicrobial treatment. Antimicrob Agents Chemother. 2007;51:1987–1994. - PMC - PubMed
-
- Peralta G, Lamelo M, Alvarez-García P, Velasco M, Delgado A, Horcajada JP, Montero M, Roiz MP, Fariñas MC, Alonso J, Martínez LM, Gutiérrez-Macías A, Alava JA, Rodríguez A, Fleites A, Navarro V, Sirvent E, Capdevila JA, SEMI- BLEE STUDY GROUP Impact of empirical treatment in extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella spp bacteremia. A multicentric cohort study. BMC Infect Dis. 2012;12:245. - PMC - PubMed
-
- Turnidge JD. The pharmacodynamics of β-lactams. Clin Infect Dis. 1998;27:10–22. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

