Endothelial cell-derived pentraxin 3 limits the vasoreparative therapeutic potential of circulating angiogenic cells
- PMID: 27659714
- PMCID: PMC5157134
- DOI: 10.1093/cvr/cvw209
Endothelial cell-derived pentraxin 3 limits the vasoreparative therapeutic potential of circulating angiogenic cells
Abstract
Aims: Circulating angiogenic cells (CACs) promote revascularization of ischaemic tissues although their underlying mechanism of action and the consequences of delivering varying number of these cells for therapy remain unknown. This study investigates molecular mechanisms underpinning CAC modulation of blood vessel formation.
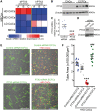
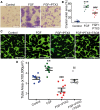
Methods and results: CACs at low (2 × 105 cells/mL) and mid (2 × 106 cells/mL) cellular densities significantly enhanced endothelial cell tube formation in vitro, while high density (HD) CACs (2 × 107 cells/mL) significantly inhibited this angiogenic process. In vivo, Matrigel-based angiogenesis assays confirmed mid-density CACs as pro-angiogenic and HD CACs as anti-angiogenic. Secretome characterization of CAC-EC conditioned media identified pentraxin 3 (PTX3) as only present in the HD CAC-EC co-culture. Recombinant PTX3 inhibited endothelial tube formation in vitro and in vivo. Importantly, our data revealed that the anti-angiogenic effect observed in HD CAC-EC co-cultures was significantly abrogated when PTX3 bioactivity was blocked using neutralizing antibodies or PTX3 siRNA in endothelial cells. We show evidence for an endothelial source of PTX3, triggered by exposure to HD CACs. In addition, we confirmed that PTX3 inhibits fibroblast growth factor (FGF) 2-mediated angiogenesis, and that the PTX3 N-terminus, containing the FGF-binding site, is responsible for such anti-angiogenic effects.
Conclusion: Endothelium, when exposed to HD CACs, releases PTX3 which markedly impairs the vascular regenerative response in an autocrine manner. Therefore, CAC density and accompanying release of angiocrine PTX3 are critical considerations when using these cells as a cell therapy for ischaemic disease.
Keywords: Cell therapy; Circulating angiogenic cell; Ischaemia; PTX3; Vascular repair.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures





References
-
- de Jong R, Houtgraaf JH, Samiei S, Boersma E, Duckers HJ. Intracoronary stem cell infusion after acute myocardial infarction: a meta-analysis and update on clinical trials. Circ Cardiovasc Interv 2014;7:156–167. - PubMed
-
- Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis 2010;209:10–17. - PubMed
-
- Taljaard M, Ward MR, Kutryk MJ, Courtman DW, Camack NJ, Goodman SG, Parker TG, Dick AJ, Galipeau J, Stewart DJ. Rationale and design of Enhanced Angiogenic Cell Therapy in Acute Myocardial Infarction (ENACT-AMI): the first randomized placebo-controlled trial of enhanced progenitor cell therapy for acute myocardial infarction. Am Heart J 2010;159:354–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

