PET and SPECT studies in children with hemispheric low-grade gliomas
- PMID: 27659825
- PMCID: PMC5120676
- DOI: 10.1007/s00381-016-3125-z
PET and SPECT studies in children with hemispheric low-grade gliomas
Abstract
Molecular imaging is playing an increasing role in the pretreatment evaluation of low-grade gliomas. While glucose positron emission tomography (PET) can be helpful to differentiate low-grade from high-grade tumors, PET imaging with amino acid radiotracers has several advantages, such as better differentiation between tumors and non-tumorous lesions, optimized biopsy targeting, and improved detection of tumor recurrence. This review provides a brief overview of single-photon emission computed tomography (SPECT) studies followed by a more detailed review of the clinical applications of glucose and amino acid PET imaging in low-grade hemispheric gliomas. We discuss key differences in the performance of the most commonly utilized PET radiotracers and highlight the advantage of PET/MRI fusion to obtain optimal information about tumor extent, heterogeneity, and metabolism. Recent data also suggest that simultaneous acquisition of PET/MR images and the combination of advanced MRI techniques with quantitative PET can further improve the pretreatment and post-treatment evaluation of pediatric brain tumors.
Keywords: Amino acid; Glucose; Low-grade; MRI; Pediatric glioma; Positron emission tomography.
Figures
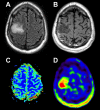
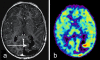
References
-
- Bagni B, Pinna L, Tamarozzi R, et al. SPET imaging of intracranial tumours with 99Tcm-sestamibi. Nucl Med Commun. 1995;16:258–64. - PubMed
-
- Black KL, Hawkins RA, Kim KT, Becker DP, Lerner C, Marciano D. Use of thallium-201 SPECT to quantitate malignancy grade of gliomas. J Neurosurg. 1989;71:342–6. - PubMed
-
- Borgwardt L, Larsen HJ, Pedersen K, Højgaard L. Practical use and implementation of PET in children in a hospital PET centre. Eur J Nucl Med Mol Imaging. 2003;30:1389–1397. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

