Intraperitoneal Meropenem for Polymicrobial Peritoneal Dialysis-Related Peritonitis
- PMID: 27659932
- PMCID: PMC5033637
- DOI: 10.3747/pdi.2016.00023
Intraperitoneal Meropenem for Polymicrobial Peritoneal Dialysis-Related Peritonitis
Abstract
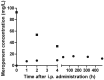
With the current rise in multiresistant gram-negative bacteria, carbapenems are more frequently used. Surprisingly, limited data exist on the pharmacokinetics of meropenem in peritoneal dialysis (PD)-related peritonitis. We report on the pharmacokinetics of repeated intraperitoneal (IP) meropenem during 21 days as treatment for polymicrobial multiresistent PD-related peritonitis.Our current report supports daily doses of 125 mg/L intraperitoneal meropenem in all bags as an effective and safe modality in the treatment of PD-associated peritonitis with multiresistant microorganisms. No signs of over- or underdosing were found based on serial drug concentration measurements at fixed time points up to 21 days.
Keywords: Carbapenems; PD-related peritonitis; meropenem.
Copyright © 2016 International Society for Peritoneal Dialysis.
Figures
References
-
- Li PK, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423. - PubMed
-
- European Committee on Antimicrobial Susceptibility Testing (EUCAST) Meropenem: Rationale for the EUCAST Clinical Breakpoints, Version 1.5. Växjö, Sweden: EUCAST; 2009. [Online.] Available at www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents.... Accessed 10 June 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

