Mammary alveolar cell as in vitro evaluation system for casein gene expression involved in glucose level
- PMID: 27660020
- PMCID: PMC5411853
- DOI: 10.5713/ajas.16.0515
Mammary alveolar cell as in vitro evaluation system for casein gene expression involved in glucose level
Abstract
Objective: Glucose is an essential fuel in the energy metabolism and synthesis pathways of all mammalian cells. In lactating animals, glucose is the major precursor for lactose and is a substrate for the synthesis of milk proteins and fat in mammary secretory (alveolar) epithelial cells. However, clear utilization of glucose in mammary cells during lactogenesis is still unknown, due to the lack of in vitro analyzing models. Therefore, the objective of this study was to test the reliability of the mammary alveolar (MAC-T) cell as an in vitro study model for glucose metabolism and lactating system.
Methods: Undifferentiated MAC-T cells were cultured in three types of Dulbecco's modified Eagle's medium with varying levels of glucose (no-glucose: 0 g/L, low-glucose: 1 g/L, and high-glucose: 4.5 g/L) for 8 d, after which differentiation to casein secretion was induced. Cell proliferation and expression levels of apoptotic genes, Insulin like growth factor-1 (IGF1) receptor, oxytocin receptor, αS1, αS2, and β casein genes were analyzed at 1, 2, 4, and 8 d after differentiation.
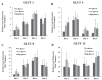
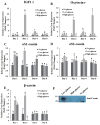
Results: The proliferation of MAC-T cells with high-glucose treatment was seen to be significantly higher. Expression of apoptotic genes was not affected in any group. However, expression levels of the mammary development related gene (IGF1 receptor) and lactation related gene (oxytocin receptor) were significantly higher in the low-glucose group. Expressions of αS1-casein, αS2-casein, and β-casein were also higher in the low-glucose treated group as compared to that in the no-glucose and high-glucose groups.
Conclusion: The results demonstrated that although a high-glucose environment increases cell proliferation in MAC-T cells, a low-glucose treatment to MAC-T cells induces higher expression of casein genes. Our results suggest that the MAC-T cells may be used as an in vitro model to analyze mammary cell development and lactation connected with precise biological effects.
Keywords: Casein; Glucose; Insulin-like Growth Factor-1 (IGF-1); Mammary alveolar (MAC-T) Cell; Oxytocin.
Conflict of interest statement
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Figures

References
-
- Cardenas H, Carvajal A, Utreras E, et al. Lactation inhibits the potentiating effect of galanin upon the GnRH-induced LH release observed in diestrous-1 rat. Biol Res. 1998;31:351–8. - PubMed
-
- Threadgold LC, Kuhn NJ. Glucose-6-phosphate hydrolysis by lactating rat mammary gland. Int J Biochem. 1979;10:683–5. - PubMed
-
- Kuhn NJ, Carrick DT, Wilde CJ. Lactose synthesis: the possibilities of regulation. J Dairy Sci. 1980;63:328–36. - PubMed
-
- Xiao CT, Cant JP. Relationship between glucose transport and metabolism in isolated bovine mammary epithelial cells. J Dairy Sci. 2005;88:2794–805. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

