Leisingera sp. JC1, a Bacterial Isolate from Hawaiian Bobtail Squid Eggs, Produces Indigoidine and Differentially Inhibits Vibrios
- PMID: 27660622
- PMCID: PMC5014874
- DOI: 10.3389/fmicb.2016.01342
Leisingera sp. JC1, a Bacterial Isolate from Hawaiian Bobtail Squid Eggs, Produces Indigoidine and Differentially Inhibits Vibrios
Abstract
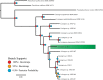
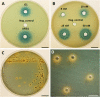
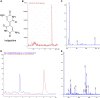
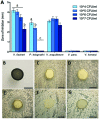
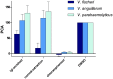
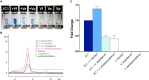
Female members of many cephalopod species house a bacterial consortium in the accessory nidamental gland (ANG), part of the reproductive system. These bacteria are deposited into eggs that are then laid in the environment where they must develop unprotected from predation, pathogens, and fouling. In this study, we characterized the genome and secondary metabolite production of Leisingera sp. JC1, a member of the roseobacter clade (Rhodobacteraceae) of Alphaproteobacteria isolated from the jelly coat of eggs from the Hawaiian bobtail squid, Euprymna scolopes. Whole genome sequencing and MLSA analysis revealed that Leisingera sp. JC1 falls within a group of roseobacters associated with squid ANGs. Genome and biochemical analyses revealed the potential for and production of a number of secondary metabolites, including siderophores and acyl-homoserine lactones involved with quorum sensing. The complete biosynthetic gene cluster for the pigment indigoidine was detected in the genome and mass spectrometry confirmed the production of this compound. Furthermore, we investigated the production of indigoidine under co-culture conditions with Vibrio fischeri, the light organ symbiont of E. scolopes, and with other vibrios. Finally, both Leisingera sp. JC1 and secondary metabolite extracts of this strain had differential antimicrobial activity against a number of marine vibrios, suggesting that Leisingera sp. JC1 may play a role in host defense against other marine bacteria either in the eggs and/or ANG. These data also suggest that indigoidine may be partially, but not wholly, responsible for the antimicrobial activity of this squid-associated bacterium.
Keywords: DART-MS; Euprymna; Leisingera; Rhodobacteraceae; indigoidine; roseobacter; secondary metabolite regulation; symbiosis.
Figures


References
-
- Amsler C. D., McClintock J. B., Baker B. J. (2001). Secondary metabolites as mediators of trophic interactions among Antarctic marine organisms. Am. Zool. 41 17–26.
-
- Barbieri E., Barry K., Child A., Wainwright N. (1997). Antimicrobial activity in the microbial community of the accessory nidamental gland and egg cases of Loligo pealei (Cephalopoda: Loliginidae). Biol. Bull. 193 275–276. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

