β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation
- PMID: 27661104
- PMCID: PMC5341812
- DOI: 10.18632/oncotarget.12119
β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation
Abstract
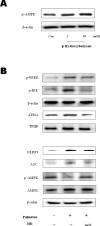
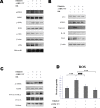
β-Hydroxybutyrate, a ketone body that is used as an energy source in organs such as the brain, muscle, and heart when blood glucose is low, is produced by fatty acid oxidation in the liver under the fasting state. Endoplasmic reticulum (ER) stress is linked with the generation of intracellular reactive oxygen species and the accumulation of misfolded protein in the ER. ER stress is known to induce the NOD-like receptor protein 3 inflammasome, which mediates activation of the proinflammatory cytokine interleukin-1β, whose maturation is caspase-1-dependent. We investigated whether β-hydroxybutyrate modulates ER stress, inflammasome formation, and insulin signaling. Sprague Dawley rats (6 and 24 months of age) that were starved for 3 d and rats treated with β-hydroxybutyrate (200 mg·kg-1·d-1 i.p., for 5 d) were used for in vivo investigations, whereas human hepatoma HepG2 cells were used for in vitro studies. Overexpression of AMPK in cultured cells was performed to elucidate the molecular mechanism. The starvation resulted in increased serum β-hydroxybutyrate levels with decreased ER stress (PERK, IRE1, and ATF6α) and inflammasome (ASC, caspase-1, and NLRP3) formation compared with non-fasted 24-month-old rats. In addition, β-hydroxybutyrate suppressed the increase of ER stress- and inflammasome-related marker proteins. Furthermore, β-hydroxybutyrate treatment increased the expression of manganese superoxide dismutase and catalase via the AMP-activated protein kinase-forkhead box protein O3α transcription factor pathway both in vivo and in vitro. The significance of the current study was the discovery of the potential therapeutic role of β-hydroxybutyrate in suppressing ER-stress-induced inflammasome formation.
Keywords: AMPK; Gerotarget; aging; endoplasmic reticulum; inflammasome; β-hydroxybutyrate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

