Development and Validation of a Photonumeric Scale for Evaluation of Transverse Neck Lines
- PMID: 27661746
- PMCID: PMC5671792
- DOI: 10.1097/DSS.0000000000000851
Development and Validation of a Photonumeric Scale for Evaluation of Transverse Neck Lines
Abstract
Background: A validated scale is needed for objective and reproducible comparisons of horizontal neck lines before and after treatment in practice and clinical studies.
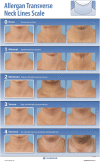
Objective: To describe the development and validation of the 5-point photonumeric Allergan Transverse Neck Lines Scale.
Methods: The Allergan Transverse Neck Lines Scale was developed to include an assessment guide, verbal descriptors, morphed images, and real subject images for each scale grade. The clinical significance of a 1-point score difference was evaluated in a review of multiple image pairs representing varying differences in severity. Interrater and intrarater reliability was evaluated in a live-subject rating validation study (N = 297) completed during 2 sessions occurring 3 weeks apart.
Results: A difference of ≥1 point on the scale was shown to reflect a clinically significant difference (mean [95% confidence interval] absolute score difference, 1.22 [1.09-1.35] for clinically different image pairs and 0.57 [0.42-0.72] for not clinically different pairs). Intrarater agreement between the 2 live-subject rating validation sessions was substantial (mean weighted kappa = 0.78). Interrater agreement was substantial during the second rating session (0.73, primary end point).
Conclusion: The Allergan Transverse Neck Lines Scale is a validated and reliable scale for rating of severity of neck lines.
Conflict of interest statement
B. Hardas, A. Marx, and D.K. Murphy are employees of Allergan plc. L. Creutz provided medical writing services at the request of the authors, which was funded by Allergan plc. The remaining authors have indicated no significant interest with commercial supporters. The opinions expressed in this article are those of the authors. The authors received no honorarium or other form of financial support related to the development of this article.
Figures

References
-
- Chao YY, Chiu HH, Howell DJ. A novel injection technique for horizontal neck lines correction using calcium hydroxylapatite. Dermatol Surg 2011;37:1542–5. - PubMed
-
- Brandt FS, Boker A. Botulinum toxin for the treatment of neck lines and neck bands. Dermatol Clin 2004;22:159–66. - PubMed
-
- Raspaldo H, Niforos FR, Gassia V, Dallara JM, et al. Lower-face and neck antiaging treatment and prevention using onabotulinumtoxin A: the 2010 multidisciplinary French consensus–part 2. J Cosmet Dermatol 2011;10:131–49. - PubMed
-
- Carruthers JD, Glogau RG, Blitzer A. Advances in facial rejuvenation: botulinum toxin type a, hyaluronic acid dermal fillers, and combination therapies–consensus recommendations. Plast Reconstr Surg 2008;121(Suppl 5):5S–30S. - PubMed
-
- Ascher B, Talarico S, Cassuto D, Escobar S, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)—Part II: wrinkles on the middle and lower face, neck and chest. J Eur Acad Dermatol Venereol 2010;24:1285–95. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

