Patient-centered methadone treatment: a randomized clinical trial
- PMID: 27661788
- PMCID: PMC5296234
- DOI: 10.1111/add.13622
Patient-centered methadone treatment: a randomized clinical trial
Abstract
Background and aims: Methadone patients who discontinue treatment are at high risk of relapse, yet a substantial proportion discontinue treatment within the first year. We investigated whether a patient-centered approach to methadone treatment improved participant outcomes at 12 months following admission, compared with methadone treatment-as-usual.
Design: Two-arm open-label randomized trial.
Setting: Two methadone treatment programs (MTPs) in Baltimore, MD, USA.
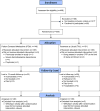
Participants: Three hundred newly admitted MTP patients were enrolled between 13 September 2011 and 26 March 2014. Their mean age was 42.7 years [standard deviation (SD) = 10.1] and 59% were males.
Intervention: Newly admitted MTP patients were assigned randomly to either patient-centered methadone treatment (PCM; n = 149), which modified the MTP's rules (e.g. counseling attendance was optional), and counselor roles (e.g. counselors were not responsible for enforcing clinic rules) or treatment-as-usual (TAU; n = 151).
Measurements: The primary outcome was opioid-positive urine test at 12-month follow-up. Other 12-month outcomes included days of heroin and cocaine use, cocaine-positive urine tests, meeting DSM-IV opioid and cocaine dependence diagnostic criteria, HIV risk behavior and quality of life and retention in treatment.
Findings: There was no significant difference between PCM and TAU conditions in opioid-positive urine screens at 12 months [adjusted odds ratio = 0.98; 95% confidence interval (CI) = 0.61, 1.56]. There were also no significant differences in any of the secondary outcome measures (all Ps > 0.05) except Quality of Life Global Score (P = 0.04; 95% CI = 0.01, 0.45). There were no significant differences between conditions in the number of individual or group counseling sessions attended. (Ps > 0.05).
Conclusions: Patient-centered methadone treatment (with optional counseling and the counselor not serving as the treatment program disciplinarian) does not appear to be more effective than methadone treatment-as-usual.
Keywords: Methadone treatment; opioid substitution therapy; opioid use disorder; patient-centered care; therapeutic alliance; treatment retention.
© 2016 Society for the Study of Addiction.
Conflict of interest statement
Declarations of interest No financial disclosures were reported by the other authors.
Figures
Comment in
-
Patient-centred care-perhaps the future of substance abuse treatment.Addiction. 2017 Mar;112(3):465-466. doi: 10.1111/add.13673. Addiction. 2017. PMID: 28168786 No abstract available.
-
Prioritizing the patient in patient-centered addictions treatment.Addiction. 2017 Mar;112(3):466-467. doi: 10.1111/add.13680. Addiction. 2017. PMID: 28168794 No abstract available.
References
-
- Darke S, Ross J, Teesson M, Ali R, Cooke R, Ritter A, et al. Factors associated with 12 months continuous heroin abstinence: findings from the Australian Treatment Outcome Study (ATOS) J Subst Abuse Treat. 2005;28:255–263. - PubMed
-
- Gossop M, Marsden J, Stewart D, Treacy S. Outcomes after methadone maintenance and methadone reduction treatments: two-year follow-up results from the National Treatment Outcome Research Study. Drug Alcohol Depend. 2001;62:255–264. - PubMed
-
- Kwiatkowski CF, Booth RE. Methadone maintenance as HIV risk reduction with street-recruited injecting drug users. J Acquir Immune Defic Syndr. 2001;26:483–489. - PubMed
-
- Simpson DD, Savage LJ, Lloyd MR. Follow-up evaluation of treatment of drug abuse during 1969 to 1972. Arch Gen Psychiatry. 1979;36:772–780. - PubMed
-
- Hubbard RL, Craddock SG, Flynn PM, Anderson J, Etheridge RM. Overview of 1-year follow-up outcomes in the Drug Abuse Treatment Outcome Study (DATOS) Psychol Addict Behav. 1997;11:261–278.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

