Splenic pooling and loss of VCAM-1 causes an engraftment defect in patients with myelofibrosis after allogeneic hematopoietic stem cell transplantation
- PMID: 27662011
- PMCID: PMC5394870
- DOI: 10.3324/haematol.2016.146811
Splenic pooling and loss of VCAM-1 causes an engraftment defect in patients with myelofibrosis after allogeneic hematopoietic stem cell transplantation
Abstract
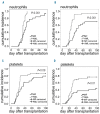
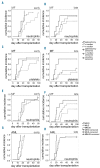
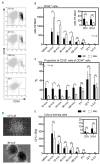
Myelofibrosis is a myeloproliferative neoplasm that results in cytopenia, bone marrow fibrosis and extramedullary hematopoiesis. Allogeneic hematopoietic stem cell transplantation is the only curative treatment but is associated with a risk of delayed engraftment and graft failure. In this study, patients with myelofibrosis (n=31) and acute myeloid leukemia (n=31) were analyzed for time to engraftment, graft failure and engraftment-related factors. Early and late neutrophil engraftment and late thrombocyte engraftment were significantly delayed in patients with myelofibrosis as compared to acute myeloid leukemia, and graft failure only occurred in myelofibrosis (6%). Only spleen size had a significant influence on engraftment efficiency in myelofibrosis patients. To analyze the cause for the engraftment defect, clearance of hematopoietic stem cells from peripheral blood was measured and immunohistological staining of bone marrow sections was performed. Numbers of circulating CD34+ were significantly reduced at early time points in myelofibrosis patients, whereas CD34+CD38- and colony-forming cells showed no significant difference in clearance. Staining of bone marrow sections for homing proteins revealed a loss of VCAM-1 in myelofibrosis with a corresponding significant increase in the level of soluble VCAM-1 within the peripheral blood. In conclusion, our data suggest that reduced engraftment and graft failure in myelofibrosis patients is caused by an early pooling of CD34+ hematopoietic stem cells in the spleen and a bone marrow homing defect caused by the loss of VCAM-1. Improved engraftment in myelofibrosis might be achieved by approaches that reduce spleen size and cleavage of VCAM-1 in these patients prior to hematopoietic stem cell transplantation.
Copyright© Ferrata Storti Foundation.
Figures


Similar articles
-
Splenic irradiation as a component of a reduced-intensity conditioning regimen for hematopoietic stem cell transplantation in myelofibrosis with massive splenomegaly.Tohoku J Exp Med. 2012 Dec;228(4):295-9. doi: 10.1620/tjem.228.295. Tohoku J Exp Med. 2012. PMID: 23117264
-
The effects of hematopoietic stem cell transplant on splenic extramedullary hematopoiesis in patients with myeloproliferative neoplasm-associated myelofibrosis.Hematol Oncol Stem Cell Ther. 2016 Sep;9(3):96-104. doi: 10.1016/j.hemonc.2016.07.002. Epub 2016 Aug 6. Hematol Oncol Stem Cell Ther. 2016. PMID: 27521149
-
Enlarged spleen is associated with low neutrophil and platelet engraftment rates and poor survival after allogeneic stem cell transplantation in patients with acute myeloid leukemia and myelodysplastic syndrome.Ann Hematol. 2018 Jun;97(6):1049-1056. doi: 10.1007/s00277-018-3278-9. Epub 2018 Feb 17. Ann Hematol. 2018. PMID: 29455235
-
Splenomegaly in patients with primary or secondary myelofibrosis who are candidates for allogeneic hematopoietic cell transplantation: a Position Paper on behalf of the Chronic Malignancies Working Party of the EBMT.Lancet Haematol. 2023 Jan;10(1):e59-e70. doi: 10.1016/S2352-3026(22)00330-1. Epub 2022 Dec 6. Lancet Haematol. 2023. PMID: 36493799 Review.
-
Allogeneic hematopoietic stem cell transplantation for myelofibrosis.Curr Opin Hematol. 2006 Mar;13(2):74-8. doi: 10.1097/01.moh.0000203191.99447.98. Curr Opin Hematol. 2006. PMID: 16456372 Review.
Cited by
-
[Impact of splenomegaly on outcomes of allogeneic hematopoietic stem cell transplantation in patients with chronic myelomonocytic leukemia].Zhonghua Xue Ye Xue Za Zhi. 2020 Apr 14;41(4):308-312. doi: 10.3760/cma.j.issn.0253-2727.2020.04.008. Zhonghua Xue Ye Xue Za Zhi. 2020. PMID: 32447935 Free PMC article. Chinese.
-
State-of-the-art review: allogeneic stem cell transplantation for myelofibrosis in 2019.Haematologica. 2019 Apr;104(4):659-668. doi: 10.3324/haematol.2018.206151. Epub 2019 Mar 14. Haematologica. 2019. PMID: 30872371 Free PMC article.
-
Pretransplant hepatomegaly is linked to relapse in patients with leukemia and myelodysplastic syndrome not in remission.Int J Hematol. 2024 Mar;119(3):316-326. doi: 10.1007/s12185-023-03707-7. Epub 2024 Jan 22. Int J Hematol. 2024. PMID: 38252235
-
2021 Update on allogeneic hematopoietic stem cell transplant for myelofibrosis: A review of current data and applications on risk stratification and management.Am J Hematol. 2021 Nov 1;96(11):1532-1538. doi: 10.1002/ajh.26349. Epub 2021 Oct 5. Am J Hematol. 2021. PMID: 34536293 Free PMC article. Review.
-
Determinants of survival in myelofibrosis patients undergoing allogeneic hematopoietic cell transplantation.Leukemia. 2021 Jan;35(1):215-224. doi: 10.1038/s41375-020-0815-z. Epub 2020 Apr 14. Leukemia. 2021. PMID: 32286544
References
-
- Titmarsh GJ, Duncombe AS, McMullin MF, et al. How common are myeloproliferative neoplasms¿ A systematic review and meta-analysis. Am J Hematol. 2014;89(6):581–587. - PubMed
-
- Mesa RA, Verstovsek S, Cervantes F, et al. Primary myelofibrosis (PMF), post polycythemia vera myelofibrosis (post-PV MF), post essential thrombocythemia myelofibrosis (post-ET MF), blast phase PMF (PMF-BP): Consensus on terminology by the international working group for myelofibrosis research and treatment (IWG-MRT). Leuk Res. 2007;31(6):737–740. - PubMed
-
- Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–1708. - PubMed
-
- Tefferi A. How I treat myelofibrosis. Blood. 2011;117(13):3494–3504. - PubMed
-
- Alchalby H, Kroger N. Reduced-intensity conditioning followed by allogeneic hematopoietic stem cell transplantation in myelofibrosis. Curr Hematol Malig Rep. 2010;5(2):53–61. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

