sRNA-Mediated Control of Transcription Termination in E. coli
- PMID: 27662085
- PMCID: PMC5040353
- DOI: 10.1016/j.cell.2016.09.004
sRNA-Mediated Control of Transcription Termination in E. coli
Abstract
Bacterial small RNAs (sRNAs) have been implicated in various aspects of post-transcriptional gene regulation. Here, we demonstrate that sRNAs also act at the level of transcription termination. We use the rpoS gene, which encodes a general stress sigma factor σ(S), as a model system, and show that sRNAs DsrA, ArcZ, and RprA bind the rpoS 5'UTR to suppress premature Rho-dependent transcription termination, both in vitro and in vivo. sRNA-mediated antitermination markedly stimulates transcription of rpoS during the transition to the stationary phase of growth, thereby facilitating a rapid adjustment of bacteria to global metabolic changes. Next generation RNA sequencing and bioinformatic analysis indicate that Rho functions as a global "attenuator" of transcription, acting at the 5'UTR of hundreds of bacterial genes, and that its suppression by sRNAs is a widespread mode of bacterial gene regulation.
Keywords: Rho; Transcription termination; antitermination; sRNA; sigma factor; stress response.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures
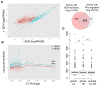




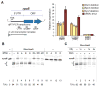

References
-
- Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EGH, Margalit H, Altuvia S. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr Biol. 2001;11:941–950. - PubMed
SUPPLEMENTAL REFERENCES
-
- Møller T, Franch T, Højrup P, Keene DR, Bächinger HP, Brennan RG, Valentin-Hansen P. Hfq: A Bacterial Sm-like Protein that Mediates RNA-RNA Interaction. Mol Cell. 2002;9:23–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

