Leishmania donovani inhibits macrophage apoptosis and pro-inflammatory response through AKT-mediated regulation of β-catenin and FOXO-1
- PMID: 27662364
- PMCID: PMC5071566
- DOI: 10.1038/cdd.2016.101
Leishmania donovani inhibits macrophage apoptosis and pro-inflammatory response through AKT-mediated regulation of β-catenin and FOXO-1
Abstract
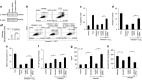
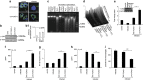
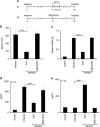
In order to establish infection, intra-macrophage parasite Leishmania donovani needs to inhibit host defense parameters like inflammatory cytokine production and apoptosis. In the present study, we demonstrate that the parasite achieves both by exploiting a single host regulator AKT for modulating its downstream transcription factors, β-catenin and FOXO-1. L. donovani-infected RAW264.7 and bone marrow-derived macrophages (BMDM) treated with AKT inhibitor or dominant negative AKT constructs showed decreased anti-inflammatory cytokine production and increased host cell apoptosis resulting in reduced parasite survival. Infection-induced activated AKT triggered phosphorylation-mediated deactivation of its downstream target, GSK-3β. Inactivated GSK-3β, in turn, could no longer sequester cytosolic β-catenin, an anti-apoptotic transcriptional regulator, as evidenced from its nuclear translocation during infection. Constitutively active GSK-3β-transfected L. donovani-infected cells mimicked the effects of AKT inhibition and siRNA-mediated silencing of β-catenin led to disruption of mitochondrial potential along with increased caspase-3 activity and IL-12 production leading to decreased parasite survival. In addition to activating anti-apoptotic β-catenin, phospho-AKT inhibits activation of FOXO-1, a pro-apoptotic transcriptional regulator. Nuclear retention of FOXO-1, inhibited during infection, was reversed when infected cells were transfected with dominant negative AKT constructs. Overexpression of FOXO-1 in infected macrophages not only documented increased apoptosis but promoted enhanced TLR4 expression and NF-κB activity along with an increase in IL-1β and decrease in IL-10 secretion. In vivo administration of AKT inhibitor significantly decreased liver and spleen parasite burden and switched cytokine balance in favor of host. In contrast, GSK-3β inhibitor did not result in any significant change in infectivity parameters. Collectively our findings revealed that L. donovani triggered AKT activation to regulate GSK-3β/β-catenin/FOXO-1 axis, thus ensuring inhibition of both host cell apoptosis and immune response essential for its intra-macrophage survival.
Figures





Similar articles
-
Crosstalk of PD-1 signaling with the SIRT1/FOXO-1 axis during the progression of visceral leishmaniasis.J Cell Sci. 2019 May 2;132(9):jcs226274. doi: 10.1242/jcs.226274. J Cell Sci. 2019. PMID: 30910830
-
Myeloid cell IL-10 production in response to leishmania involves inactivation of glycogen synthase kinase-3β downstream of phosphatidylinositol-3 kinase.J Immunol. 2012 Jan 1;188(1):367-78. doi: 10.4049/jimmunol.1100076. Epub 2011 Dec 2. J Immunol. 2012. PMID: 22140263
-
Leishmania donovani Exploits Myeloid Cell Leukemia 1 (MCL-1) Protein to Prevent Mitochondria-dependent Host Cell Apoptosis.J Biol Chem. 2016 Feb 12;291(7):3496-507. doi: 10.1074/jbc.M115.672873. Epub 2015 Dec 15. J Biol Chem. 2016. PMID: 26670606 Free PMC article.
-
Cadmium inhibits neural stem/progenitor cells proliferation via MitoROS-dependent AKT/GSK-3β/β-catenin signaling pathway.J Appl Toxicol. 2021 Dec;41(12):1998-2010. doi: 10.1002/jat.4179. Epub 2021 May 11. J Appl Toxicol. 2021. PMID: 33977565 Review.
-
Probing the molecular mechanism of aggressive infection by antimony resistant Leishmania donovani.Cytokine. 2021 Sep;145:155245. doi: 10.1016/j.cyto.2020.155245. Epub 2020 Aug 26. Cytokine. 2021. PMID: 32861564 Review.
Cited by
-
Leishmania Hijacks Myeloid Cells for Immune Escape.Front Microbiol. 2018 May 7;9:883. doi: 10.3389/fmicb.2018.00883. eCollection 2018. Front Microbiol. 2018. PMID: 29867798 Free PMC article. Review.
-
Gasdermin-D activation promotes NLRP3 activation and host resistance to Leishmania infection.Nat Commun. 2023 Feb 24;14(1):1049. doi: 10.1038/s41467-023-36626-6. Nat Commun. 2023. PMID: 36828815 Free PMC article.
-
Systems biology of autophagy in leishmanial infection and its diverse role in precision medicine.Front Mol Biosci. 2023 Apr 21;10:1113249. doi: 10.3389/fmolb.2023.1113249. eCollection 2023. Front Mol Biosci. 2023. PMID: 37152895 Free PMC article. Review.
-
Differential immune response modulation in early Leishmania amazonensis infection of BALB/c and C57BL/6 macrophages based on transcriptome profiles.Sci Rep. 2019 Dec 27;9(1):19841. doi: 10.1038/s41598-019-56305-1. Sci Rep. 2019. PMID: 31882833 Free PMC article.
-
Role of glutathione, ROS, and Bcl-xL in the inhibition of apoptosis of monocyte-derived dendritic cells by Leishmania mexicana promastigotes.Parasitol Res. 2018 Apr;117(4):1225-1235. doi: 10.1007/s00436-018-5804-z. Epub 2018 Feb 23. Parasitol Res. 2018. PMID: 29476339
References
-
- Neves BM, Silvestre R, Resende M, Ouaissi A, Cunha J, Tavares J et al. Activation of phosphatidylinositol 3-kinase/Akt and impairment of nuclear factor-kappaB: molecular mechanisms behind the arrested maturation/activation state of Leishmania infantum-infected dendritic cells. Am J Pathol 2010; 177: 2898–2911. - PMC - PubMed
-
- Ruhland A, Leal N, Kima PE. Leishmania promastigotes activate PI3K/Akt signalling to confer host cell resistance to apoptosis. Cell Microbiol 2007; 9: 84–96. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

