Low-Osmolar Diet and Adjusted Water Intake for Vasopressin Reduction in Autosomal Dominant Polycystic Kidney Disease: A Pilot Randomized Controlled Trial
- PMID: 27663039
- PMCID: PMC5123924
- DOI: 10.1053/j.ajkd.2016.07.023
Low-Osmolar Diet and Adjusted Water Intake for Vasopressin Reduction in Autosomal Dominant Polycystic Kidney Disease: A Pilot Randomized Controlled Trial
Abstract
Background: Autosomal dominant polycystic kidney disease (ADPKD) affects millions of people worldwide. Vasopressin promotes disease progression.
Study design: A randomized controlled trial with equal (1:1) allocation.
Setting & participants: This trial examined the effect of combining a low-osmolar (low-sodium [1,500mg/d], low-protein [0.8g per kilogram of body weight]) diet and adjusted water intake on vasopressin secretion in 34 patients with ADPKD.
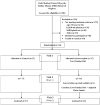
Intervention: Participants were randomly assigned to receive a low-osmolar diet followed by adjusted water intake to achieve urine osmolality ≤ 280mOsm/kg water versus no intervention for 2 weeks.
Outcome: The primary outcome of the study was change (delta) in copeptin levels and urine osmolality between the intervention and control groups from baseline to 2 weeks.
Measurements: Fasting plasma copeptin level, 24-hour urine osmolality, and total solute intake.
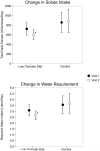
Results: Baseline characteristics of the 2 groups were similar. Mean plasma copeptin levels and urine osmolality declined from 6.2±3.05 (SD) to 5.3±2.5pmol/L (P=0.02) and from 426±193 to 258±117mOsm/kg water (P=0.01), respectively, in the intervention group compared to a nonsignificant change in the control group (from 4.7±3.6 to 5.07±4pmol/L [P=0.2] and 329±159 to 349±139mOsm/kg water [P=0.3], respectively). The change in copeptin levels (primary outcome) and urine osmolality was statistically significant between the intervention and control groups (delta copeptin, -0.86±1.3 vs +0.39±1.2pmol/L [P=0.009]; delta urine osmolality, -167±264 vs +20±80mOsm/kg water [P=0.007], respectively). Total urinary solute decreased in only the intervention group and significantly differed between groups at week 1 (P=0.03), reducing mean water prescription from 3.2 to 2.6L/d.
Limitations: Small sample size and short follow-up.
Conclusions: We developed a stepwise dietary intervention that led to a significant reduction in vasopressin secretion in patients with ADPKD. Furthermore, this intervention led to a reduction in water required for vasopressin reduction.
Keywords: Autosomal dominant polycystic kidney disease (ADPKD); clinical trial; copeptin; nutrition; osmolality; protein; salt; urea; vasopressin; water.
Copyright © 2016 National Kidney Foundation, Inc. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Grantham J, Torres VE. Progression of autosomal dominant polycystic kidney disease (ADPKD) to renal failure. In: Seldin DW, Geibisch G, editors. The Kidney: Physiology and Pathophysiology. Lippincott Williams and Wilkins; Philadelphia: 2000. pp. 2513–36. CB.
-
- Perrone R, Ruthazer R, Terrin N. Survival after end-stage renal disease in autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2001;38:777–84. - PubMed
-
- Gabow PA, Kaehny WD, Johnson AM, Duley IT, Manco-Johnson M, Lezotte DC, et al. The clinical utility of renal concentrating capacity in polycystic kidney disease. Kidney international. 1989;35(2):675–80. - PubMed
-
- Zittema D, Boertien WE, van Beek AP, Dullaart RP, Franssen CF, de Jong PE, et al. Vasopressin, copeptin, and renal concentrating capacity in patients with autosomal dominant polycystic kidney disease without renal impairment. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(6):906–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

