Regulating the regulator: Insights into the cardiac protein phosphatase 1 interactome
- PMID: 27663175
- PMCID: PMC5154861
- DOI: 10.1016/j.yjmcc.2016.09.009
Regulating the regulator: Insights into the cardiac protein phosphatase 1 interactome
Abstract
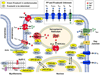
Reversible phosphorylation of proteins is a delicate yet dynamic balancing act between kinases and phosphatases, the disturbance of which underlies numerous disease processes. While our understanding of protein kinases has grown tremendously over the past decades, relatively little is known regarding protein phosphatases. This may be because protein kinases are great in number and relatively specific in function, and thereby amenable to be studied in isolation, whereas protein phosphatases are much less abundant and more nonspecific in their function. To achieve subcellular localization and substrate specificity, phosphatases depend on partnering with a large number of regulatory subunits, protein scaffolds and/or other interactors. This added layer of complexity presents a significant barrier to their study, but holds the key to unexplored opportunities for novel pharmacologic intervention. In this review we focus on serine/threonine protein phosphatase type-1 (PP1), which plays an important role in cardiac physiology and pathophysiology. Although much work has been done to investigate the role of PP1 in cardiac diseases including atrial fibrillation and heart failure, most of these studies were limited to examining and manipulating the catalytic subunit(s) of PP1 without adequately considering the PP1 interactors, which give specificity to PP1's functions. To complement these studies, three unbiased methods have been developed and applied to the mapping of the PP1 interactome: bioinformatics approaches, yeast two-hybrid screens, and affinity-purification mass spectrometry. The application of these complementary methods has the potential to generate a detailed cardiac PP1 interactome, which is an important step in identifying novel and targeted pharmacological interventions.
Keywords: Atrial fibrillation; Heart failure; Protein phosphatase; Proteomics; Regulatory subunit.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
There are no relevant relationships with industry.
Figures

Similar articles
-
Alterations in the interactome of serine/threonine protein phosphatase type-1 in atrial fibrillation patients.J Am Coll Cardiol. 2015 Jan 20;65(2):163-73. doi: 10.1016/j.jacc.2014.10.042. J Am Coll Cardiol. 2015. PMID: 25593058 Free PMC article.
-
Rearrangement of the Protein Phosphatase 1 Interactome During Heart Failure Progression.Circulation. 2018 Oct 9;138(15):1569-1581. doi: 10.1161/CIRCULATIONAHA.118.034361. Circulation. 2018. PMID: 29669786 Free PMC article.
-
Impaired local regulation of ryanodine receptor type 2 by protein phosphatase 1 promotes atrial fibrillation.Cardiovasc Res. 2014 Jul 1;103(1):178-87. doi: 10.1093/cvr/cvu123. Epub 2014 May 8. Cardiovasc Res. 2014. PMID: 24812280 Free PMC article.
-
Function and regulation of phosphatase 1 in healthy and diseased heart.Cell Signal. 2022 Feb;90:110203. doi: 10.1016/j.cellsig.2021.110203. Epub 2021 Nov 22. Cell Signal. 2022. PMID: 34822978 Review.
-
Complex functionality of protein phosphatase 1 isoforms in the heart.Cell Signal. 2021 Sep;85:110059. doi: 10.1016/j.cellsig.2021.110059. Epub 2021 May 29. Cell Signal. 2021. PMID: 34062239 Review.
Cited by
-
Mechanisms underlying pathological Ca2+ handling in diseases of the heart.Pflugers Arch. 2021 Mar;473(3):331-347. doi: 10.1007/s00424-020-02504-z. Epub 2021 Jan 5. Pflugers Arch. 2021. PMID: 33399957 Free PMC article. Review.
-
Proteomics of the heart.Physiol Rev. 2024 Jul 1;104(3):931-982. doi: 10.1152/physrev.00026.2023. Epub 2024 Feb 1. Physiol Rev. 2024. PMID: 38300522 Free PMC article. Review.
-
Differentially expressed genes for atrial fibrillation identified by RNA sequencing from paired human left and right atrial appendages.Physiol Genomics. 2019 Aug 1;51(8):323-332. doi: 10.1152/physiolgenomics.00012.2019. Epub 2019 Jun 7. Physiol Genomics. 2019. PMID: 31172864 Free PMC article.
-
Promise of adeno-associated virus as a gene therapy vector for cardiovascular diseases.Heart Fail Rev. 2017 Nov;22(6):795-823. doi: 10.1007/s10741-017-9622-7. Heart Fail Rev. 2017. PMID: 28589503 Review.
-
Cardiac myosin binding protein-C phosphorylation as a function of multiple protein kinase and phosphatase activities.Nat Commun. 2024 Jun 14;15(1):5111. doi: 10.1038/s41467-024-49408-5. Nat Commun. 2024. PMID: 38877002 Free PMC article.
References
-
- Shi Y. Serine/threonine phosphatases: mechanism through structure. Cell. 2009;139:468–484. - PubMed
-
- Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol. Rev. 2004;84:1–39. - PubMed
-
- Heroes E, Lesage B, Gornemann J, Beullens M, Van Meervelt L, Bollen M. The PP1 binding code: a molecular-lego strategy that governs specificity. FEBS J. 2013;280:584–595. - PubMed
-
- Christ T, Boknik P, Wohrl S, Wettwer E, Graf EM, Bosch RF, et al. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–2657. - PubMed
-
- El-Armouche A, Boknik P, Eschenhagen T, Carrier L, Knaut M, Ravens U, et al. Molecular determinants of altered Ca2+ handling in human chronic atrial fibrillation. Circulation. 2006;114:670–680. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

