Visualisation of Kiss1 Neurone Distribution Using a Kiss1-CRE Transgenic Mouse
- PMID: 27663274
- PMCID: PMC5091624
- DOI: 10.1111/jne.12435
Visualisation of Kiss1 Neurone Distribution Using a Kiss1-CRE Transgenic Mouse
Abstract
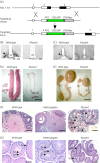
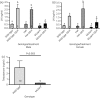
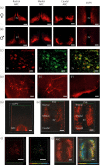
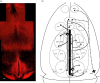
Kisspeptin neuropeptides are encoded by the Kiss1 gene and play a critical role in the regulation of the mammalian reproductive axis. Kiss1 neurones are found in two locations in the rodent hypothalamus: one in the arcuate nucleus (ARC) and another in the RP3V region, which includes the anteroventral periventricular nucleus (AVPV). Detailed mapping of the fibre distribution of Kiss1 neurones will help with our understanding of the action of these neurones in other regions of the brain. We have generated a transgenic mouse in which the Kiss1 coding region is disrupted by a CRE-GFP transgene so that expression of the CRE recombinase protein is driven from the Kiss1 promoter. As expected, mutant mice of both sexes are sterile with hypogonadotrophic hypogonadism and do not show the normal rise in luteinising hormone after gonadectomy. Mutant female mice do not develop mature Graafian follicles or form corpora lutea consistent with ovulatory failure. Mutant male mice have low blood testosterone levels and impaired spermatogenesis beyond the meiosis stage. Breeding Kiss-CRE heterozygous mice with CRE-activated tdTomato reporter mice allows fluorescence visualisation of Kiss1 neurones in brain slices. Approximately 80-90% of tdTomato positive neurones in the ARC were co-labelled with kisspeptin and expression of tdTomato in the AVPV region was sexually dimorphic, with higher expression in females than males. A small number of tdTomato-labelled neurones was also found in other locations, including the lateral septum, the anterodorsal preoptic nucleus, the amygdala, the dorsomedial and ventromedial hypothalamic nuclei, the periaquaductal grey, and the mammillary nucleus. Three dimensional visualisation of Kiss1 neurones and fibres by CLARITY processing of whole brains showed an increase in ARC expression during puberty and higher numbers of Kiss1 neurones in the caudal region of the ARC compared to the rostral region. ARC Kiss1 neurones sent fibre projections to several hypothalamic regions, including rostrally to the periventricular and pre-optic areas and to the lateral hypothalamus.
Keywords: CLARITY; Kiss-CRE; mouse; neuronal distribution; tdTomato; transgenic.
© 2016 The Authors. Journal of Neuroendocrinology published by John Wiley & Sons Ltd on behalf of British Society for Neuroendocrinology.
Figures

Similar articles
-
Deficiency of arcuate nucleus kisspeptin results in postpubertal central hypogonadism.Am J Physiol Endocrinol Metab. 2021 Aug 1;321(2):E264-E280. doi: 10.1152/ajpendo.00088.2021. Epub 2021 Jun 28. Am J Physiol Endocrinol Metab. 2021. PMID: 34181485 Free PMC article.
-
Mapping neuronal inputs to Kiss1 neurons in the arcuate nucleus of the mouse.PLoS One. 2019 Mar 27;14(3):e0213927. doi: 10.1371/journal.pone.0213927. eCollection 2019. PLoS One. 2019. PMID: 30917148 Free PMC article.
-
Sex difference in developmental changes in visualized Kiss1 neurons in newly generated Kiss1-Cre rats.J Reprod Dev. 2023 Oct 20;69(5):227-238. doi: 10.1262/jrd.2023-019. Epub 2023 Jul 28. J Reprod Dev. 2023. PMID: 37518187 Free PMC article.
-
Gonadal and nongonadal regulation of sex differences in hypothalamic Kiss1 neurones.J Neuroendocrinol. 2010 Jul;22(7):682-91. doi: 10.1111/j.1365-2826.2010.02030.x. Epub 2010 May 12. J Neuroendocrinol. 2010. PMID: 20492362 Free PMC article. Review.
-
Sex steroids and the control of the Kiss1 system: developmental roles and major regulatory actions.J Neuroendocrinol. 2012 Jan;24(1):22-33. doi: 10.1111/j.1365-2826.2011.02230.x. J Neuroendocrinol. 2012. PMID: 21951227 Review.
Cited by
-
Transcriptome-scale spatial gene expression in rat arcuate nucleus during puberty.Cell Biosci. 2022 Jan 21;12(1):8. doi: 10.1186/s13578-022-00745-2. Cell Biosci. 2022. PMID: 35063020 Free PMC article.
-
Dominant Neuropeptide Cotransmission in Kisspeptin-GABA Regulation of GnRH Neuron Firing Driving Ovulation.J Neurosci. 2018 Jul 11;38(28):6310-6322. doi: 10.1523/JNEUROSCI.0658-18.2018. Epub 2018 Jun 13. J Neurosci. 2018. PMID: 29899026 Free PMC article.
-
Estrogen differentially regulates transcriptional landscapes of preoptic and arcuate kisspeptin neuron populations.Front Endocrinol (Lausanne). 2022 Aug 25;13:960769. doi: 10.3389/fendo.2022.960769. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36093104 Free PMC article.
-
Medial Amygdala Kiss1 Neurons Mediate Female Pheromone Stimulation of Luteinizing Hormone in Male Mice.Neuroendocrinology. 2019;108(3):172-189. doi: 10.1159/000496106. Epub 2018 Dec 10. Neuroendocrinology. 2019. PMID: 30537700 Free PMC article.
-
The expression of IGFBP-5 in the reproductive axis and effect on the onset of puberty in female rats.Reprod Biol Endocrinol. 2022 Jul 12;20(1):100. doi: 10.1186/s12958-022-00966-7. Reprod Biol Endocrinol. 2022. PMID: 35821045 Free PMC article.
References
-
- Colledge WH, Doran J, Mei H. Model systems for studying kisspeptin signalling: mice and cells. Adv Exp Med Biol 2013; 784: 481–503. - PubMed
-
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005; 146: 4431–4436. - PubMed
-
- Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology 2011; 152: 1001–1012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

