Eligibility of real-life patients with COPD for inclusion in trials of inhaled long-acting bronchodilator therapy
- PMID: 27663386
- PMCID: PMC5034631
- DOI: 10.1186/s12931-016-0433-5
Eligibility of real-life patients with COPD for inclusion in trials of inhaled long-acting bronchodilator therapy
Abstract
Background: Management guidelines of chronic obstructive pulmonary disease (COPD) are mainly based on results of randomised controlled trials (RCTs), but some authors have suggested limited representativeness of patients included in these trials. No previous studies have applied the full range of selection criteria to a broad COPD patient population in a real-life setting.
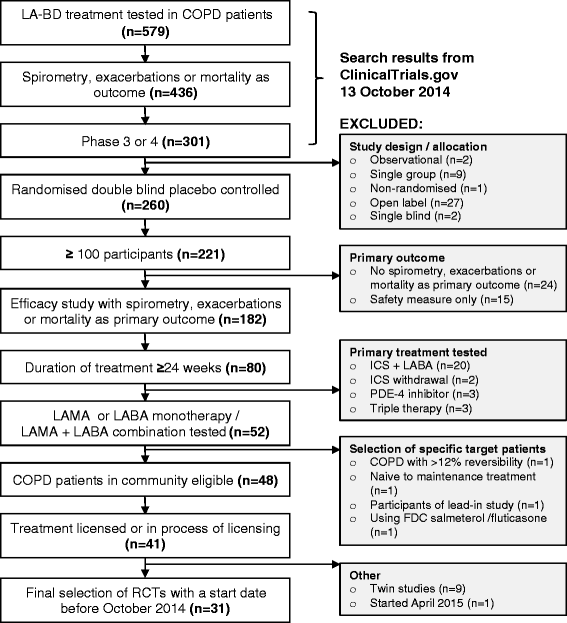
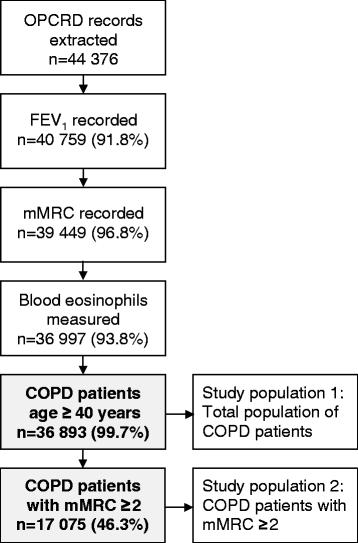
Methods: We identified all RCTs of inhaled long-acting bronchodilator therapy, during 1999-2013, at ClinicalTrials.gov and translated trial selection criteria into definitions compatible with electronic medical records. Eligibility was calculated for each RCT by applying these criteria to a uniquely representative, well-characterised population of patients with COPD from the Optimum Patient Care Research Database (OPCRD).
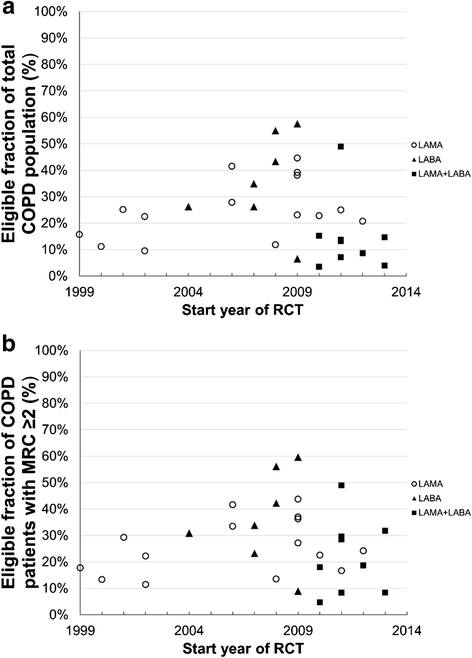
Results: Median eligibility of 36 893 patients with COPD for participation in 31 RCTs was 23 % (interquartile range 12-38). Two studies of olodaterol showed the highest eligibility of 55 and 58 %. Conversely, the lowest eligibility was observed in two studies that required a history of exacerbations in the past year (3.5 and 3.9 %). For the patient subgroup with modified Medical Research Council score ≥2, the overall median eligibility was 27 %.
Conclusions: By applying an extensive range of RCT selection criteria to a large, representative COPD patient population, this study highlights that the interpretation of results from RCTs must take into account that RCT participants are variably, but generally more representative of patients in the community than previously believed.
Keywords: Chronic obstructive pulmonary disease; Long-acting bronchodilator; Randomised controlled trial; Real-life research.
Figures
References
-
- Disease GIfCOL . Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2016. p. 112.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

