A Rice Ca2+ Binding Protein Is Required for Tapetum Function and Pollen Formation
- PMID: 27663411
- PMCID: PMC5100779
- DOI: 10.1104/pp.16.01261
A Rice Ca2+ Binding Protein Is Required for Tapetum Function and Pollen Formation
Abstract
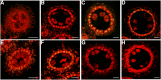
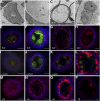
In flowering plants, successful male reproduction requires the sophisticated interaction between somatic anther wall layers and reproductive cells. Timely degradation of the innermost tissue of the anther wall layer, the tapetal layer, is critical for pollen development. Ca2+ is a well-known stimulus for plant development, but whether it plays a role in affecting male reproduction remains elusive. Here we report a role of Defective in Exine Formation 1 (OsDEX1) in rice (Oryza sativa), a Ca2+ binding protein, in regulating rice tapetal cell degradation and pollen formation. In osdex1 anthers, tapetal cell degeneration is delayed and degradation of the callose wall surrounding the microspores is compromised, leading to aborted pollen formation and complete male sterility. OsDEX1 is expressed in tapetal cells and microspores during early anther development. Recombinant OsDEX1 is able to bind Ca2+ and regulate Ca2+ homeostasis in vitro, and osdex1 exhibited disturbed Ca2+ homeostasis in tapetal cells. Phylogenetic analysis suggested that OsDEX1 may have a conserved function in binding Ca2+ in flowering plants, and genetic complementation of pollen wall defects of an Arabidopsis (Arabidopsis thaliana) dex1 mutant confirmed its evolutionary conservation in pollen development. Collectively, these findings suggest that OsDEX1 plays a fundamental role in the development of tapetal cells and pollen formation, possibly via modulating the Ca2+ homeostasis during pollen development.
© 2016 American Society of Plant Biologists. All Rights Reserved.
Figures








Similar articles
-
PERSISTENT TAPETAL CELL1 encodes a PHD-finger protein that is required for tapetal cell death and pollen development in rice.Plant Physiol. 2011 Jun;156(2):615-30. doi: 10.1104/pp.111.175760. Epub 2011 Apr 22. Plant Physiol. 2011. PMID: 21515697 Free PMC article.
-
OsGPAT3 Plays a Critical Role in Anther Wall Programmed Cell Death and Pollen Development in Rice.Int J Mol Sci. 2018 Dec 12;19(12):4017. doi: 10.3390/ijms19124017. Int J Mol Sci. 2018. PMID: 30545137 Free PMC article.
-
PERSISTENT TAPETAL CELL2 Is Required for Normal Tapetal Programmed Cell Death and Pollen Wall Patterning.Plant Physiol. 2020 Feb;182(2):962-976. doi: 10.1104/pp.19.00688. Epub 2019 Nov 26. Plant Physiol. 2020. PMID: 31772077 Free PMC article.
-
Rice anther tapetum: a vital reproductive cell layer for sporopollenin biosynthesis and pollen exine patterning.Plant Biol (Stuttg). 2023 Mar;25(2):233-245. doi: 10.1111/plb.13485. Epub 2022 Dec 16. Plant Biol (Stuttg). 2023. PMID: 36350096 Review.
-
The biosynthesis, composition and assembly of the outer pollen wall: A tough case to crack.Phytochemistry. 2015 May;113:170-82. doi: 10.1016/j.phytochem.2014.05.002. Epub 2014 Jun 3. Phytochemistry. 2015. PMID: 24906292 Review.
Cited by
-
Exploiting Genic Male Sterility in Rice: From Molecular Dissection to Breeding Applications.Front Plant Sci. 2021 Mar 2;12:629314. doi: 10.3389/fpls.2021.629314. eCollection 2021. Front Plant Sci. 2021. PMID: 33763090 Free PMC article. Review.
-
OsPKS2 is required for rice male fertility by participating in pollen wall formation.Plant Cell Rep. 2018 May;37(5):759-773. doi: 10.1007/s00299-018-2265-x. Epub 2018 Feb 6. Plant Cell Rep. 2018. PMID: 29411094
-
Cytological Analysis and Fine Mapping of paa1 (Post-meiosis Abnormal Anther 1) Mutant with Abnormal Tapetum and Microspore Development.Biochem Genet. 2022 Dec;60(6):2268-2285. doi: 10.1007/s10528-022-10217-4. Epub 2022 Mar 24. Biochem Genet. 2022. PMID: 35325440
-
Calreticulin expression and localization in relation to exchangeable Ca2+ during pollen development in Petunia.BMC Plant Biol. 2022 Jan 8;22(1):24. doi: 10.1186/s12870-021-03409-4. BMC Plant Biol. 2022. PMID: 34998378 Free PMC article.
-
Integrated Analysis of Microarray, Small RNA, and Degradome Datasets Uncovers the Role of MicroRNAs in Temperature-Sensitive Genic Male Sterility in Wheat.Int J Mol Sci. 2022 Jul 22;23(15):8057. doi: 10.3390/ijms23158057. Int J Mol Sci. 2022. PMID: 35897633 Free PMC article.
References
-
- Abeele FV, Skryma R, Shuba Y, Van Coppenolle F, Slomianny C, Roudbaraki M, Mauroy B, Wuytack F, Prevarskaya N (2002) Bcl-2-dependent modulation of Ca2+ homeostasis and store-operated channels in prostate cancer cells. Cancer Cell 1: 169–179 - PubMed
-
- Aditya J, Lewis J, Shirley NJ, Tan HT, Henderson M, Fincher GB, Burton RA, Mather DE, Tucker MR (2015) The dynamics of cereal cyst nematode infection differ between susceptible and resistant barley cultivars and lead to changes in (1,3;1,4)-β-glucan levels and HvCslF gene transcript abundance. New Phytol 207: 135–147 - PubMed
-
- Ariizumi T, Hatakeyama K, Hinata K, Inatsugi R, Nishida I, Sato S, Kato T, Tabata S, Toriyama K (2004) Disruption of the novel plant protein NEF1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J 39: 170–181 - PubMed
-
- Ariizumi T, Toriyama K (2011) Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol 62: 437–460 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

