Promise and limits of the CellSearch platform for evaluating pharmacodynamics in circulating tumor cells
- PMID: 27663478
- PMCID: PMC5074690
- DOI: 10.1053/j.seminoncol.2016.06.004
Promise and limits of the CellSearch platform for evaluating pharmacodynamics in circulating tumor cells
Abstract
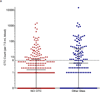
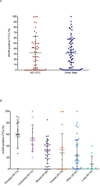
Circulating tumor cells (CTCs), which are captured from blood with anti-epithelial cell adhesion molecule (EpCAM) antibodies, have established prognostic value in specific epithelial cancers, but less is known about their utility for assessing patient response to molecularly targeted agents via measurement of pharmacodynamic (PD) endpoints. We discuss the use of CellSearch (Janssen Diagnostics, LLC, Raritan, NJ) CTC isolation technology for monitoring PD response in early phase trials. We present representative data from three clinical trials with the poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888) suggesting that CTCs can be used to measure PD effects. However, while often leading to hypothesis-generating information, our experience points to the difficulty in obtaining sufficient EpCAM-expressing CTCs from patients with advanced disease to reach statistically significant conclusions about PD effects from each trial. Overall, the level of phenotypic heterogeneity observed in specimens from patients with advanced carcinomas suggests caution in the use of cell-surface differentiation marker-based methods for isolating CTCs.
Keywords: Assay validation; CTCs; Pharmacodynamics; Surrogate endpoint.
Copyright © 2016 Elsevier Inc. All rights reserved.
Conflict of interest statement
None
Figures







References
-
- Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. - PubMed
-
- Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. - PubMed
-
- de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. - PubMed
-
- Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. - PubMed
-
- Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–4224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

