Alternative Watson-Crick Synthetic Genetic Systems
- PMID: 27663774
- PMCID: PMC5088529
- DOI: 10.1101/cshperspect.a023770
Alternative Watson-Crick Synthetic Genetic Systems
Abstract
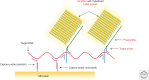
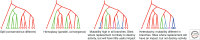
In its "grand challenge" format in chemistry, "synthesis" as an activity sets out a goal that is substantially beyond current theoretical and technological capabilities. In pursuit of this goal, scientists are forced across uncharted territory, where they must answer unscripted questions and solve unscripted problems, creating new theories and new technologies in ways that would not be created by hypothesis-directed research. Thus, synthesis drives discovery and paradigm changes in ways that analysis cannot. Described here are the products that have arisen so far through the pursuit of one grand challenge in synthetic biology: Recreate the genetics, catalysis, evolution, and adaptation that we value in life, but using genetic and catalytic biopolymers different from those that have been delivered to us by natural history on Earth. The outcomes in technology include new diagnostic tools that have helped personalize the care of hundreds of thousands of patients worldwide. In science, the effort has generated a fundamentally different view of DNA, RNA, and how they work.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
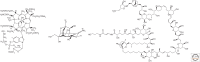





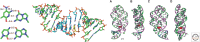





References
-
- Bain JD, Chamberlin AR, Switzer CY, Benner SA. 1992. Ribosome-mediated incorporation of non-standard amino acids into a peptide through expansion of the genetic code. Nature 356: 537–539. - PubMed
-
- Bartel DP, Szostak JW. 1993. Isolation of new ribozymes from a large pool of random sequences. Science 261: 1411–1418. - PubMed
-
- Benner SA. 2004. Understanding nucleic acids using synthetic chemistry. Accounts Chem Res 37: 784–797. - PubMed
-
- Benner SA. 2009. The life, the universe and the scientific method. FfAME, Gainesville, FL.
-
- Benner SA, Hutter D. 2002. Phosphates, DNA, and the search for nonterrean life. A second generation model for genetic molecules. Bioorg Chem 30: 62–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
