Development and validation of a novel single nucleotide polymorphism (SNP) panel for genetic analysis of Blastomyces spp. and association analysis
- PMID: 27663837
- PMCID: PMC5035486
- DOI: 10.1186/s12879-016-1847-x
Development and validation of a novel single nucleotide polymorphism (SNP) panel for genetic analysis of Blastomyces spp. and association analysis
Abstract
Background: Single nucleotide polymorphism (SNP) genotyping is increasingly being utilized for molecular typing of pathogens and is cost-effective, especially for large numbers of isolates. The goals of this study were 1) to develop and validate a SNP assay panel for genetic analysis of Blastomyces spp., 2) ascertain whether microsatellite genotyping and the SNP genotyping with the developed panel resolve identical genetic groups, and 3) explore the utility of SNPs for examining phylogenetic and virulence questions in humans.
Methods: Three hundred sixty unique Blastomyces spp. isolates previously genotyped with microsatellite markers were genotyped with the MassARRAY® SNP genotyping system (Agena Bioscience™, San Diego, CA), for a custom panel of 28 SNPs. Clinical presentation data was analyzed for association with SNP variants.
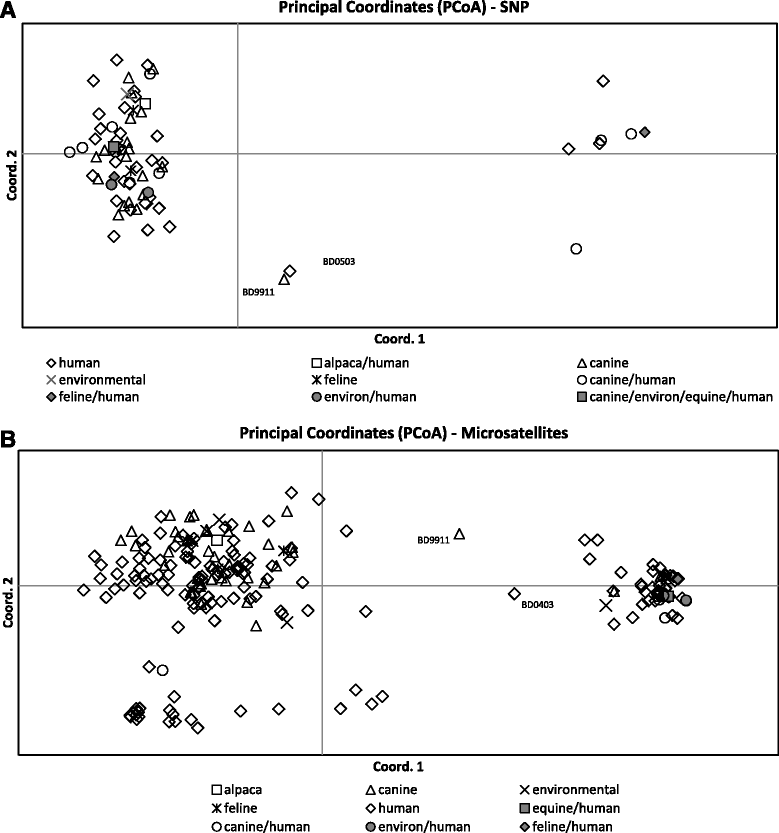
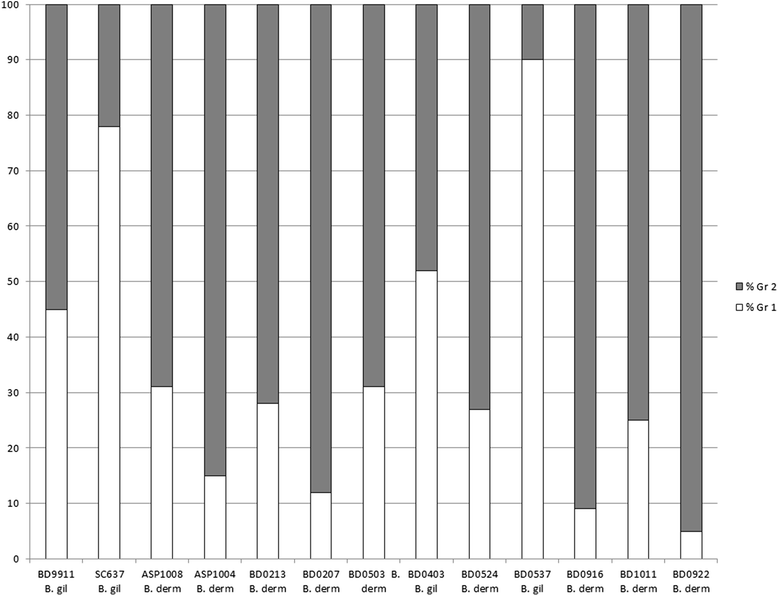
Results: Three hundred twenty-three Blastomyces spp. isolates (90 %) were successfully genotyped by SNP analysis, with results obtained for at least 27 of 28 assays. For 99.7 % of isolates tested by both genotyping methods, microsatellite genetic group assignment correlated with species assignment based on internal transcribed spacer 2 (ITS2) genotyping, with Group 1 (Gr 1) being equivalent to B. gilchristii and Group 2 (Gr 2) being equivalent to B. dermatitidis. Thirteen isolates were genetic hybrids by one or both methods of genotyping and were difficult to assign to a particular genetic group or species. Fifteen SNP loci showed significantly different alleles in cases of pulmonary vs disseminated disease, at a p-value of <0.01 or less.
Conclusions: This study is the largest genotyping study of Blastomyces spp. isolates and presents a new method for genetic analysis with which to further explore the relationship between the genetic diversity in Blastomyces spp. and clinical disease presentation. We demonstrated that microsatellite Gr 1 is equivalent to B. gilchristii and Gr 2 is equivalent to B. dermatitidis. We also discovered potential evidence of infrequent recombination between the two Blastomyces spp. Several Blastomyces spp. SNPs were identified as associated with dissemination or pulmonary disease presentation, but additional work is needed to examine virulence SNPs separately within B. dermatitidis and B. gilchristii.
Keywords: B. dermatitidis; B. gilchristii; Blastomyces; Blastomycosis; Genotype; Microsatellite; SNP.
Figures
References
-
- Kwon-Chung K, Bennett J. Medical mycology. 2. Philadelphia: Lea & Febiger; 1992. pp. 248–279.
-
- Mandell G, Bennett J, Dolin R. Principles and practice of infectious diseases. 6. New York: Churchill Livingston; 2000. pp. 2733–2746.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

