Atomoxetine and Parent Training for Children With Autism and Attention-Deficit/Hyperactivity Disorder: A 24-Week Extension Study
- PMID: 27663942
- PMCID: PMC5108566
- DOI: 10.1016/j.jaac.2016.06.015
Atomoxetine and Parent Training for Children With Autism and Attention-Deficit/Hyperactivity Disorder: A 24-Week Extension Study
Abstract
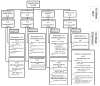
Objective: The authors previously reported on a 2-by-2 randomized clinical trial of individual and combined treatment with atomoxetine (ATX) and parent training (PT) for attention-deficit/hyperactivity disorder (ADHD) symptoms and behavioral noncompliance in 128 5- to 14-year-old children with autism spectrum disorder. In the present report, they describe a 24-week extension of treatment responders and nonresponders.
Method: One-hundred seventeen participants from the acute trial (91%) entered the extension; 84 of these were in 2 subgroups: "treatment responders" (n = 43) from all 4 groups in the acute trial, seen monthly for 24 weeks, and "placebo nonresponders" (n = 41), treated with open-label ATX for 10 weeks. Participants originally assigned to PT continued PT during the extension; the remainder served as controls. Primary outcome measurements were the parent-rated Swanson, Nolan and Pelham ADHD scale and the Home Situations Questionnaire.
Results: Sixty percent (26 of 43) of treatment responders in the acute trial, including 68% of responders originally assigned to ATX, still met the response criteria at the end of the extension. The response rate of placebo nonresponders treated with 10-week open-label ATX was 37% (15 of 41), similar to the acute trial. Children receiving open-label ATX + PT were significantly more likely to be ADHD responders (53% versus 23%) and noncompliance responders (58% versus 14%) than those receiving open-label ATX alone.
Conclusion: Most ATX responders maintained their responses during the extension. PT combined with ATX in the open-label trial appeared to improve ADHD and noncompliance outcomes more than ATX alone. Clinical trial registration information-Atomoxetine, Placebo and Parent Management Training in Autism (Strattera); http://clinicaltrials.gov; NCT00844753.
Keywords: atomoxetine; attention-deficit/hyperactivity disorder; autism; combined modality therapy; outcome assessment.
Copyright © 2016 American Academy of Child and Adolescent Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Kaat AJ, Lecavalier L. Disruptive behavior disorders in children and adolescents with autism spectrum disorders: A review of the prevalence, presentation, and treatment. Research in Autism Spectrum Disorders. 2013;7:1579–1594.
-
- Lecavalier L, Leone S, Wiltz J. The impact of behavior problems on caregiver stress in young people with autism spectrum disorders. J Intell Disabil Res. 2006;50:172–183. - PubMed
-
- Sikora DM, Vora P, Coury DL, Rosenberg D. Attention-deficit/hyperactivity disorder symptoms, adaptive functioning, and quality of life in children with autism spectrum disorder. Pediatrics. 2012;130:S91–S97. - PubMed
-
- Research Units on Pediatric Psychopharmacology Autism Network A randomized, double-blind, placebo-controlled, crossover trial of methylphenidate in children with hyperactivity associated with pervasive developmental disorders. Arch General Psychiat. 2005;62:1266–1274. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous