Andrographolide induces Nrf2 and heme oxygenase 1 in astrocytes by activating p38 MAPK and ERK
- PMID: 27663973
- PMCID: PMC5034653
- DOI: 10.1186/s12974-016-0723-3
Andrographolide induces Nrf2 and heme oxygenase 1 in astrocytes by activating p38 MAPK and ERK
Abstract
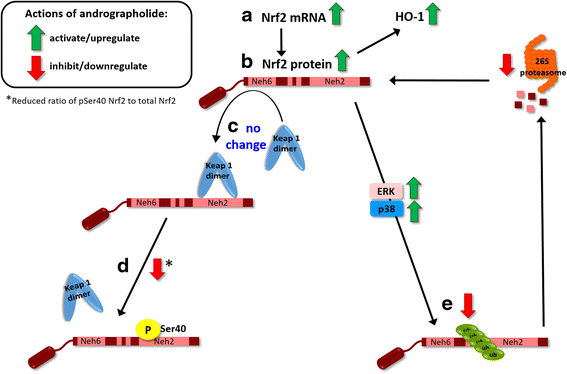
Background: Andrographolide is the major labdane diterpenoid originally isolated from Andrographis paniculata and has been shown to have anti-inflammatory and antioxidative effects. However, there is a dearth of studies on the potential therapeutic utility of andrographolide in neuroinflammatory conditions. Here, we aimed to investigate the mechanisms underlying andrographolide's effect on the expression of anti-inflammatory and antioxidant heme oxygenase-1 (HO-1) in primary astrocytes.
Methods: Measurements of the effects of andrograholide on antioxidant HO-1 and its transcription factor, Nrf2, include gene expression, protein turnover, and activation of putative signaling regulators.
Results: Andrographolide potently activated Nrf2 and also upregulated HO-1 expression in primary astrocytes. Andrographolide's effects on Nrf2 seemed to be biphasic, with acute (within 1 h) reductions in Nrf2 ubiquitination efficiency and turnover rate, followed by upregulation of Nrf2 mRNA between 8 and 24 h. The acute regulation of Nrf2 by andrographolide seemed to be independent of Keap1 and partly mediated by p38 MAPK and ERK signaling.
Conclusions: These data provide further insights into the mechanisms underlying andrographolide's effects on astrocyte-mediated antioxidant, and anti-inflammatory responses and support the further assessment of andrographolide as a potential therapeutic for neurological conditions in which oxidative stress and neuroinflammation are implicated.
Keywords: Andrographolide; Antioxidant response; Astrocyte; Heme oxygenase 1; Mitogen-activated protein kinases; Nrf2.
Figures




Similar articles
-
Andrographolide inhibits hypoxia-induced hypoxia-inducible factor 1α and endothelin 1 expression through the heme oxygenase 1/CO/cGMP/MKP-5 pathways in EA.hy926 cells.Environ Toxicol. 2018 Mar;33(3):269-279. doi: 10.1002/tox.22514. Epub 2017 Nov 22. Environ Toxicol. 2018. PMID: 29165873
-
Andrographolide stimulates p38 mitogen-activated protein kinase-nuclear factor erythroid-2-related factor 2-heme oxygenase 1 signaling in primary cerebral endothelial cells for definite protection against ischemic stroke in rats.Transl Res. 2016 Apr;170:57-72. doi: 10.1016/j.trsl.2015.12.002. Epub 2015 Dec 17. Transl Res. 2016. PMID: 26746802
-
Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells.Br J Pharmacol. 2014 Jan;171(1):237-52. doi: 10.1111/bph.12440. Br J Pharmacol. 2014. PMID: 24117426 Free PMC article.
-
Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress.J Adv Res. 2021 Jul 6;34:43-63. doi: 10.1016/j.jare.2021.06.023. eCollection 2021 Dec. J Adv Res. 2021. PMID: 35024180 Free PMC article. Review.
-
Andrographolide, a Natural Antioxidant: An Update.Antioxidants (Basel). 2019 Nov 20;8(12):571. doi: 10.3390/antiox8120571. Antioxidants (Basel). 2019. PMID: 31756965 Free PMC article. Review.
Cited by
-
Andrographolide Derivatives Target the KEAP1/NRF2 Axis and Possess Potent Anti-SARS-CoV-2 Activity.ChemMedChem. 2022 Mar 4;17(5):e202100732. doi: 10.1002/cmdc.202100732. Epub 2022 Jan 31. ChemMedChem. 2022. PMID: 35099120 Free PMC article.
-
Butein Inhibits Oxidative Stress Injury in Rats with Chronic Heart Failure via ERK/Nrf2 Signaling.Cardiovasc Ther. 2022 Jan 4;2022:8684014. doi: 10.1155/2022/8684014. eCollection 2022. Cardiovasc Ther. 2022. PMID: 35069800 Free PMC article.
-
Sulforaphane suppresses carcinogenesis of colorectal cancer through the ERK/Nrf2‑UDP glucuronosyltransferase 1A metabolic axis activation.Oncol Rep. 2020 Apr;43(4):1067-1080. doi: 10.3892/or.2020.7495. Epub 2020 Feb 10. Oncol Rep. 2020. PMID: 32323779 Free PMC article.
-
Acute dose of melatonin via Nrf2 dependently prevents acute ethanol-induced neurotoxicity in the developing rodent brain.J Neuroinflammation. 2018 Apr 21;15(1):119. doi: 10.1186/s12974-018-1157-x. J Neuroinflammation. 2018. PMID: 29679979 Free PMC article.
-
Regulation of the NRF2 transcription factor by andrographolide and organic extracts from plant endophytes.PLoS One. 2018 Oct 1;13(10):e0204853. doi: 10.1371/journal.pone.0204853. eCollection 2018. PLoS One. 2018. PMID: 30273379 Free PMC article.
References
-
- Freeman LC, Ting JP. The pathogenic role of the inflammasome in neurodegenerative diseases. J Neurochem. 2016;136 Suppl 1:29-38. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

