Mutant IDH1 and thrombosis in gliomas
- PMID: 27664011
- PMCID: PMC5640980
- DOI: 10.1007/s00401-016-1620-7
Mutant IDH1 and thrombosis in gliomas
Abstract
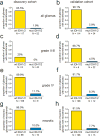
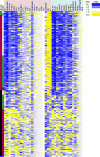
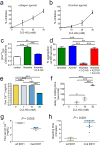
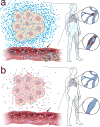
Mutant isocitrate dehydrogenase 1 (IDH1) is common in gliomas, and produces D-2-hydroxyglutarate (D-2-HG). The full effects of IDH1 mutations on glioma biology and tumor microenvironment are unknown. We analyzed a discovery cohort of 169 World Health Organization (WHO) grade II-IV gliomas, followed by a validation cohort of 148 cases, for IDH1 mutations, intratumoral microthrombi, and venous thromboemboli (VTE). 430 gliomas from The Cancer Genome Atlas were analyzed for mRNAs associated with coagulation, and 95 gliomas in a tissue microarray were assessed for tissue factor (TF) protein. In vitro and in vivo assays evaluated platelet aggregation and clotting time in the presence of mutant IDH1 or D-2-HG. VTE occurred in 26-30 % of patients with wild-type IDH1 gliomas, but not in patients with mutant IDH1 gliomas (0 %). IDH1 mutation status was the most powerful predictive marker for VTE, independent of variables such as GBM diagnosis and prolonged hospital stay. Microthrombi were far less common within mutant IDH1 gliomas regardless of WHO grade (85-90 % in wild-type versus 2-6 % in mutant), and were an independent predictor of IDH1 wild-type status. Among all 35 coagulation-associated genes, F3 mRNA, encoding TF, showed the strongest inverse relationship with IDH1 mutations. Mutant IDH1 gliomas had F3 gene promoter hypermethylation, with lower TF protein expression. D-2-HG rapidly inhibited platelet aggregation and blood clotting via a novel calcium-dependent, methylation-independent mechanism. Mutant IDH1 glioma engraftment in mice significantly prolonged bleeding time. Our data suggest that mutant IDH1 has potent antithrombotic activity within gliomas and throughout the peripheral circulation. These findings have implications for the pathologic evaluation of gliomas, the effect of altered isocitrate metabolism on tumor microenvironment, and risk assessment of glioma patients for VTE.
Keywords: D-2-hydroxyglutarate; Glioma; Isocitrate dehydrogenase; Platelet; Thrombosis; Tissue factor.
Figures

References
-
- Ades S, Kumar S, Alam M, Goodwin A, Weckstein D, Dugan M, Ashikaga T, Evans M, Verschraegen C, Holmes CE. Tumor oncogene (KRAS) status and risk of venous thrombosis in patients with metastatic colorectal cancer. J Thromb Haemost. 2015;13:998–1003. - PubMed
-
- Anand M, Brat DJ. Oncogenic regulation of tissue factor and thrombosis in cancer. Thromb Res. 2012;(129 Suppl 1):S46–S49. - PubMed
-
- Bastida E, Ordinas A, Escolar G, Jamieson GA. Tissue factor in microvesicles shed from U87MG human glioblastoma cells induces coagulation, platelet aggregation, and thrombogenesis. Blood. 1984;64:177–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

