Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis
- PMID: 27664155
- PMCID: PMC5035515
- DOI: 10.3402/jev.v5.31427
Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis
Abstract
The lymphatic system works in close collaboration with the cardiovascular system to preserve fluid balance throughout the body and is essential for the trafficking of antigen-presenting cells and lymphocytes to lymphoid organs. Recent findings have associated lymphatic dysfunction with the pathogenesis of cardiovascular-related diseases such as atherosclerosis, inflammation and obesity. Whether lymphatic dysfunction is a cause or a consequence of these diseases, as well as how, is under intensive investigation. Extracellular vesicles (EVs) are submicron vesicles released by diverse cell types upon activation or apoptosis and are considered important biomarkers for several inflammatory diseases. Thus, it is critical to characterize the presence of EVs in various biological tissues and fluids to delineate their origins and, subsequently, their functions. In the past few years, new techniques allowing the quantitative and qualitative analysis of EVs have emerged, thus facilitating the onset of studies bridging these vesicles to the lymphatic system. Using several state-of-the-art approaches, this article reports the presence of diverse EVs inclusively derived from red blood cells and platelets in lymph of healthy animals. Our results suggest that lymph from atherosclerotic mice displays a higher concentration of EVs, bringing forward the concept that EVs contained in lymph could either be a biomarker for lymphatic dysfunction or, conversely, for inflammatory disease progression.
Keywords: atherosclerosis; biomarkers; inflammation; lymphatic vessels; platelets.
Conflict of interest statement
and funding The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures

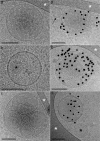

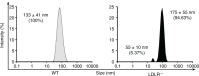

References
-
- Tammela T, Alitalo K. Lymphangiogenesis: molecular mechanisms and future promise. Cell. 2010;140:460–76. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

