Caveolins and caveolae in ocular physiology and pathophysiology
- PMID: 27664379
- PMCID: PMC5237608
- DOI: 10.1016/j.preteyeres.2016.09.005
Caveolins and caveolae in ocular physiology and pathophysiology
Abstract
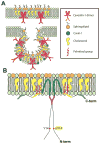
Caveolae are specialized, invaginated plasma membrane domains that are defined morphologically and by the expression of signature proteins called, caveolins. Caveolae and caveolins are abundant in a variety of cell types including vascular endothelium, glia, and fibroblasts where they play critical roles in transcellular transport, endocytosis, mechanotransduction, cell proliferation, membrane lipid homeostasis, and signal transduction. Given these critical cellular functions, it is surprising that ablation of the caveolae organelle does not result in lethality suggesting instead that caveolae and caveolins play modulatory roles in cellular homeostasis. Caveolar components are also expressed in ocular cell types including retinal vascular cells, Müller glia, retinal pigment epithelium (RPE), conventional aqueous humor outflow cells, the corneal epithelium and endothelium, and the lens epithelium. In the eye, studies of caveolae and other membrane microdomains (i.e., "lipid rafts") have lagged behind what is a substantial body of literature outside vision science. However, interest in caveolae and their molecular components has increased with accumulating evidence of important roles in vision-related functions such as blood-retinal barrier homeostasis, ocular inflammatory signaling, pathogen entry at the ocular surface, and aqueous humor drainage. The recent association of CAV1/2 gene loci with primary open angle glaucoma and intraocular pressure has further enhanced the need to better understand caveolar functions in the context of ocular physiology and disease. Herein, we provide the first comprehensive review of literature on caveolae, caveolins, and other membrane domains in the context of visual system function. This review highlights the importance of caveolae domains and their components in ocular physiology and pathophysiology and emphasizes the need to better understand these important modulators of cellular function.
Keywords: Blood-retinal barrier; Caveolae; Caveolin; Glaucoma; Lipid rafts; Neuroinflammation; Ocular hypertension; Vascular permeability.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures





References
-
- Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. - PubMed
-
- Albinsson S, Nordstrom I, Sward K, Hellstrand P. Differential dependence of stretch and shear stress signaling on caveolin-1 in the vascular wall. Am J Physiol Cell Physiol. 2008;294:C271–279. - PubMed
-
- Albinsson S, Shakirova Y, Rippe A, Baumgarten M, Rosengren BI, Rippe C, Hallmann R, Hellstrand P, Rippe B, Sward K. Arterial remodeling and plasma volume expansion in caveolin-1-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1222–1231. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

