Circadian regulation of auditory function
- PMID: 27665709
- PMCID: PMC5364078
- DOI: 10.1016/j.heares.2016.08.018
Circadian regulation of auditory function
Abstract
The circadian system integrates environmental cues to regulate physiological functions in a temporal fashion. The suprachiasmatic nucleus, located in the hypothalamus, is the master clock that synchronizes central and peripheral organ clocks to orchestrate physiological functions. Recently, molecular clock machinery has been identified in the cochlea unravelling the potential involvement in the circadian regulation of auditory functions. Here, we present background information on the circadian system and review the recent findings that introduce circadian rhythms to the auditory field. Understanding the mechanisms by which circadian rhythms regulate auditory function will provide fundamental knowledge on the signalling networks that control vulnerability and resilience to auditory insults.
Copyright © 2016 Elsevier B.V. All rights reserved.
Figures
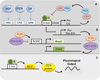
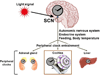



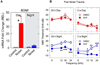

References
-
- Akhtar RA, Reddy AB, Maywood ES, Clayton JD, King VM, Smith AG, Gant TW, Hastings MH, Kyriacou CP. Circadian cycling of the mouse liver transcriptome, as revealed by cDNA microarray, is driven by the suprachiasmatic nucleus. Curr Biol. 2002;12:540–550. - PubMed
-
- Albrecht U. Invited Review: Regulation of mammalian circadian clock genes. Journal of Applied Physiology. 2002;92:1348–1355. - PubMed
-
- Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. - PubMed
-
- Brown S, Azzi A. Peripheral Circadian Oscillators in Mammals. In: Kramer A, Merrow M, editors. Circadian Clocks. Vol. 217. Springer Berlin Heidelberg; 2013. pp. 45–66. - PubMed
-
- Buchanan TW, Brechtel A, Sollers JJ, Lovallo WR. Exogenous cortisol exerts effects on the startle reflex independent of emotional modulation. Pharmacol Biochem Behav. 2001;68:203–210. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

