Meiotic cohesin subunits RAD21L and REC8 are positioned at distinct regions between lateral elements and transverse filaments in the synaptonemal complex of mouse spermatocytes
- PMID: 27665783
- PMCID: PMC5177981
- DOI: 10.1262/jrd.2016-127
Meiotic cohesin subunits RAD21L and REC8 are positioned at distinct regions between lateral elements and transverse filaments in the synaptonemal complex of mouse spermatocytes
Abstract
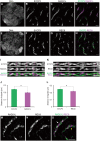
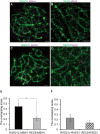
Cohesins containing a meiosis-specific α-kleisin subunit, RAD21L or REC8, play roles in diverse aspects of meiotic chromosome dynamics including formation of axial elements (AEs), assembly of the synaptonemal complex (SC), recombination of homologous chromosomes (homologs), and cohesion of sister chromatids. However, the exact functions of individual α-kleisins remain to be elucidated. Here, we examined the localization of RAD21L and REC8 within the SC by super-resolution microscopy, 3D-SIM. We found that both RAD21L and REC8 were localized at the connection sites between lateral elements (LEs) and transverse filaments (TFs) of pachynema with RAD21L locating interior to REC8 sites. RAD21L and REC8 were not symmetrical in terms of synaptic homologs, suggesting that the arrangement of different cohesins is not strictly fixed along all chromosome axes. Intriguingly, some RAD21L signals, but not REC8 signals, were observed between unsynapsed regions of AEs of zygonema as if they formed a bridge between homologs. Furthermore, the signals of recombination intermediates overlapped with those of RAD21L to a greater degree than with those of REC8. These results highlight the different properties of two meiotic α-kleisins, and strongly support the previous proposition that RAD21L is an atypical cohesin that establishes the association between homologs rather than sister chromatids.
Figures



Similar articles
-
Genetic Interactions Between the Meiosis-Specific Cohesin Components, STAG3, REC8, and RAD21L.G3 (Bethesda). 2016 Jun 1;6(6):1713-24. doi: 10.1534/g3.116.029462. G3 (Bethesda). 2016. PMID: 27172213 Free PMC article.
-
Meiotic cohesin REC8 marks the axial elements of rat synaptonemal complexes before cohesins SMC1beta and SMC3.J Cell Biol. 2003 Mar 3;160(5):657-70. doi: 10.1083/jcb.200212080. J Cell Biol. 2003. PMID: 12615909 Free PMC article.
-
Analysis of absolute amounts of two meiotic cohesin subunits, RAD21L and REC8, in mouse spermatocytes.J Reprod Dev. 2023 Apr 3;69(2):78-86. doi: 10.1262/jrd.2022-075. Epub 2023 Feb 3. J Reprod Dev. 2023. PMID: 36740274 Free PMC article.
-
Rec8 Cohesin: A Structural Platform for Shaping the Meiotic Chromosomes.Genes (Basel). 2022 Jan 22;13(2):200. doi: 10.3390/genes13020200. Genes (Basel). 2022. PMID: 35205245 Free PMC article. Review.
-
Many functions of the meiotic cohesin.Chromosome Res. 2010 Dec;18(8):909-24. doi: 10.1007/s10577-010-9169-0. Epub 2010 Nov 18. Chromosome Res. 2010. PMID: 21086039 Review.
Cited by
-
Ectopic expression of meiotic cohesin RAD21L promotes adjacency of homologous chromosomes in somatic cells.J Reprod Dev. 2017 Jun 21;63(3):227-234. doi: 10.1262/jrd.2016-171. Epub 2017 Feb 26. J Reprod Dev. 2017. PMID: 28239026 Free PMC article.
-
CpG site methylation regulates mouse Rec8 gene promoter activity.J Reprod Dev. 2025 Jun 6;71(3):145-153. doi: 10.1262/jrd.2024-077. Epub 2025 Apr 4. J Reprod Dev. 2025. PMID: 40189260 Free PMC article.
-
Canonical and noncanonical roles of Hop1 are crucial for meiotic prophase in the fungus Sordaria macrospora.PLoS Biol. 2024 Jul 1;22(7):e3002705. doi: 10.1371/journal.pbio.3002705. eCollection 2024 Jul. PLoS Biol. 2024. PMID: 38950075 Free PMC article.
-
Rad21l1 cohesin subunit is dispensable for spermatogenesis but not oogenesis in zebrafish.PLoS Genet. 2021 Jun 17;17(6):e1009127. doi: 10.1371/journal.pgen.1009127. eCollection 2021 Jun. PLoS Genet. 2021. PMID: 34138874 Free PMC article.
-
Mapping separase-mediated cleavage in situ.NAR Genom Bioinform. 2022 Nov 18;4(4):lqac085. doi: 10.1093/nargab/lqac085. eCollection 2022 Dec. NAR Genom Bioinform. 2022. PMID: 36415827 Free PMC article.
References
-
- Jordan P. Initiation of homologous chromosome pairing during meiosis. Biochem Soc Trans 2006; 34: 545–549. - PubMed
-
- Page SL, Hawley RS. The genetics and molecular biology of the synaptonemal complex. Annu Rev Cell Dev Biol 2004; 20: 525–558. - PubMed
-
- Keeney S. Mechanism and control of meiotic recombination initiation. Curr Top Dev Biol 2001; 52: 1–53. - PubMed
-
- Keeney S, Giroux CN, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 1997; 88: 375–384. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

