Zinc(II) Binding Site to the Amyloid-β Peptide: Insights from Spectroscopic Studies with a Wide Series of Modified Peptides
- PMID: 27665863
- PMCID: PMC5069684
- DOI: 10.1021/acs.inorgchem.6b01733
Zinc(II) Binding Site to the Amyloid-β Peptide: Insights from Spectroscopic Studies with a Wide Series of Modified Peptides
Abstract
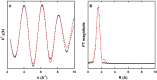
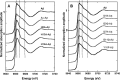
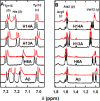
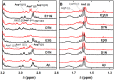
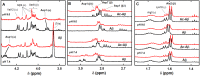
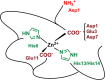
The Zn(II) ion has been linked to Alzheimer's disease (AD) due to its ability to modulate the aggregating properties of the amyloid-β (Aβ) peptide, where Aβ aggregation is a central event in the etiology of the disease. Delineating Zn(II) binding properties to Aβ is thus a prerequisite to better grasp its potential role in AD. Because of (i) the flexibility of the Aβ peptide, (ii) the multiplicity of anchoring sites, and (iii) the silent nature of the Zn(II) ion in most classical spectroscopies, this is a difficult task. To overcome these difficulties, we have investigated the impact of peptide alterations (mutations, N-terminal acetylation) on the Zn(Aβ) X-ray absorption spectroscopy fingerprint and on the Zn(II)-induced modifications of the Aβ peptides' NMR signatures. We propose a tetrahedrally bound Zn(II) ion, in which the coordination sphere is made by two His residues and two carboxylate side chains. Equilibria between equivalent ligands for one Zn(II) binding position have also been observed, the predominant site being made by the side chains of His6, His13 or His14, Glu11, and Asp1 or Glu3 or Asp7, with a slight preference for Asp1.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Kozlowski H.; Luczkowski M.; Remelli M.; Valensin D. Copper, Zinc and iron in neurodegenerative diseases (Alzheimer’s disease, Parkinson’s disease and prion diseases). Coord. Chem. Rev. 2012, 256, 2129–2141. 10.1016/j.ccr.2012.03.013. - DOI
-
- Hureau C. Coordination of redox active metal ions to the APP and to the amyloid-β peptides involved in AD. Part 1: an overview. Coord. Chem. Rev. 2012, 256, 2164–2174. 10.1016/j.ccr.2012.03.037. - DOI
-
- Viles J. H. Metal ions and amyloid formation in neurodegenerative diseases. Coord. Chem. Rev. 2012, 256, 2271–2284. 10.1016/j.ccr.2012.05.003. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

